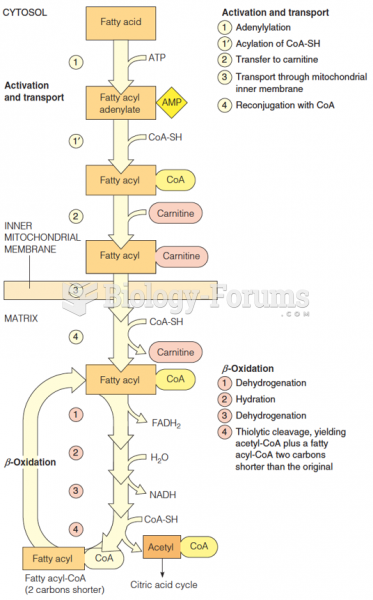
|
|
|
Did you know?
Bacteria have flourished on the earth for over three billion years. They were the first life forms on the planet.
Did you know?
Human stomach acid is strong enough to dissolve small pieces of metal such as razor blades or staples.
Did you know?
Pubic lice (crabs) are usually spread through sexual contact. You cannot catch them by using a public toilet.
Did you know?
Although not all of the following muscle groups are commonly used, intramuscular injections may be given into the abdominals, biceps, calves, deltoids, gluteals, laterals, pectorals, quadriceps, trapezoids, and triceps.
Did you know?
Of the estimated 2 million heroin users in the United States, 600,000–800,000 are considered hardcore addicts. Heroin addiction is considered to be one of the hardest addictions to recover from.