This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
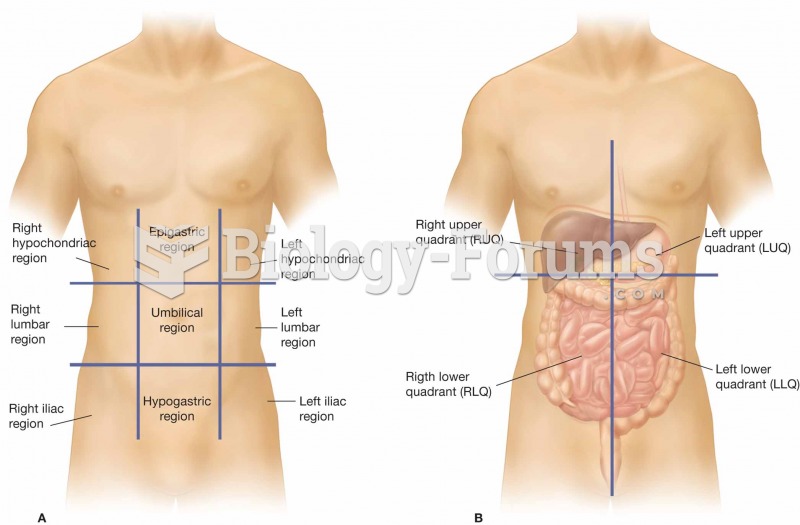
The heart is located in the center of the chest, with part of it tipped slightly so that it taps against the left side of the chest.
Did you know?
People about to have surgery must tell their health care providers about all supplements they take.
Did you know?
According to the Migraine Research Foundation, migraines are the third most prevalent illness in the world. Women are most affected (18%), followed by children of both sexes (10%), and men (6%).
Did you know?
Green tea is able to stop the scent of garlic or onion from causing bad breath.
Did you know?
According to the FDA, adverse drug events harmed or killed approximately 1,200,000 people in the United States in the year 2015.