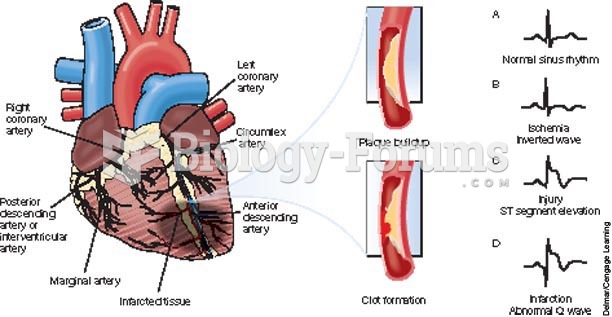
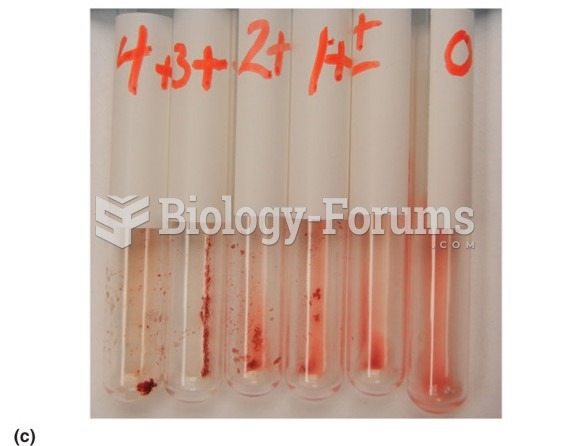
|
|
|
In the ancient and medieval periods, dysentery killed about ? of all babies before they reach 12 months of age. The disease was transferred through contaminated drinking water, because there was no way to adequately dispose of sewage, which contaminated the water.
The people with the highest levels of LDL are Mexican American males and non-Hispanic black females.
Colchicine is a highly poisonous alkaloid originally extracted from a type of saffron plant that is used mainly to treat gout.
There are 60,000 miles of blood vessels in every adult human.
Acetaminophen (Tylenol) in overdose can seriously damage the liver. It should never be taken by people who use alcohol heavily; it can result in severe liver damage and even a condition requiring a liver transplant.
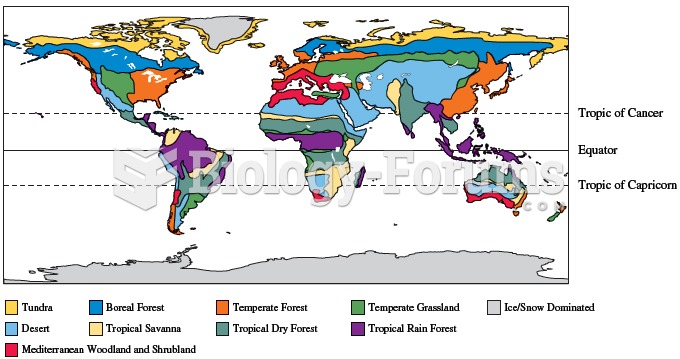 Variation in climatic conditions result in geographic variation in the distribution of biome types a
Variation in climatic conditions result in geographic variation in the distribution of biome types a
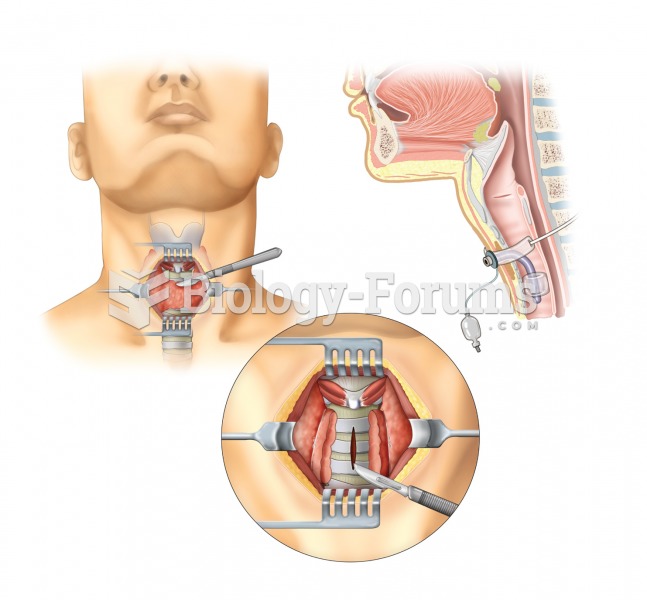 Tracheostomy. A tracheotomy, or incision into the trachea, is performed to create an opening into th
Tracheostomy. A tracheotomy, or incision into the trachea, is performed to create an opening into th