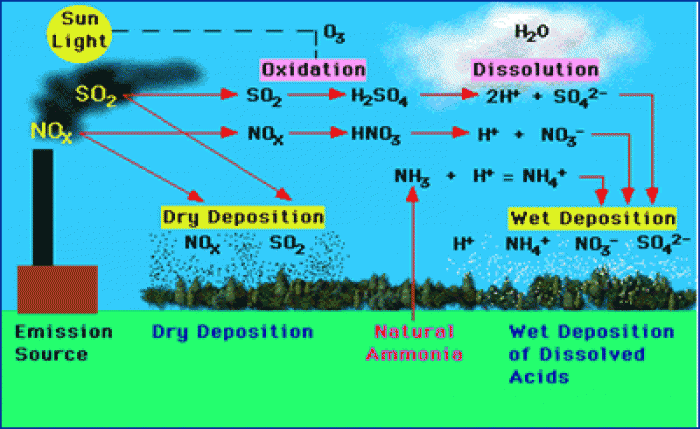
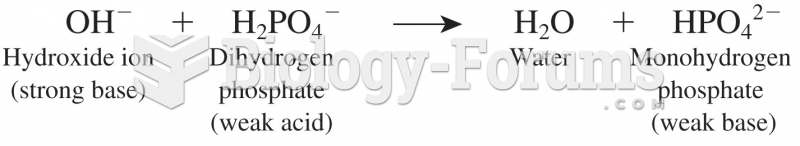
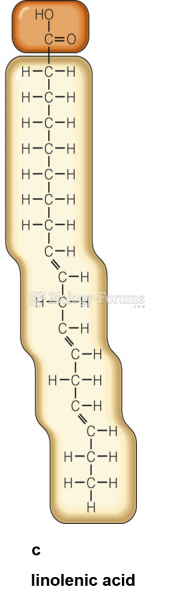
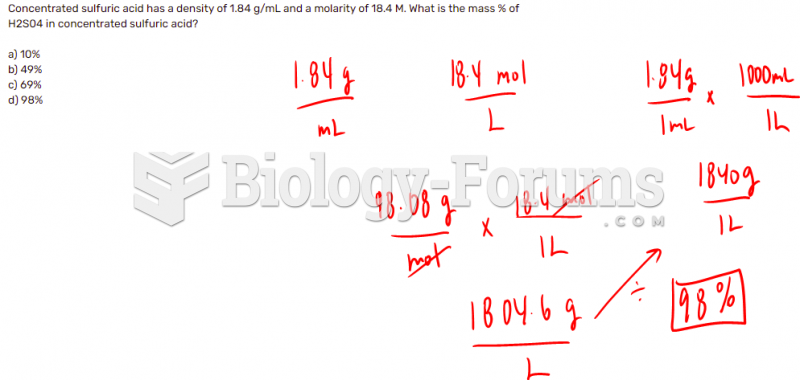
|
|
|
To maintain good kidney function, you should drink at least 3 quarts of water daily. Water dilutes urine and helps prevent concentrations of salts and minerals that can lead to kidney stone formation. Chronic dehydration is a major contributor to the development of kidney stones.
More than 34,000 trademarked medication names and more than 10,000 generic medication names are in use in the United States.
About 3.2 billion people, nearly half the world population, are at risk for malaria. In 2015, there are about 214 million malaria cases and an estimated 438,000 malaria deaths.
Nearly 31 million adults in America have a total cholesterol level that is more than 240 mg per dL.
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.