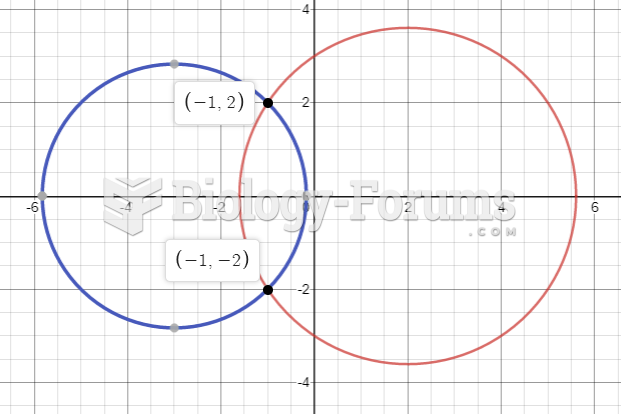
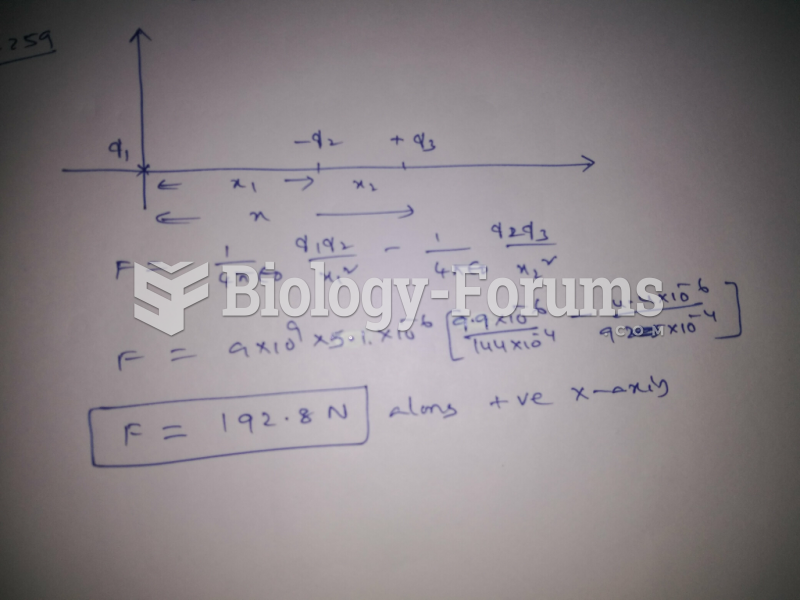
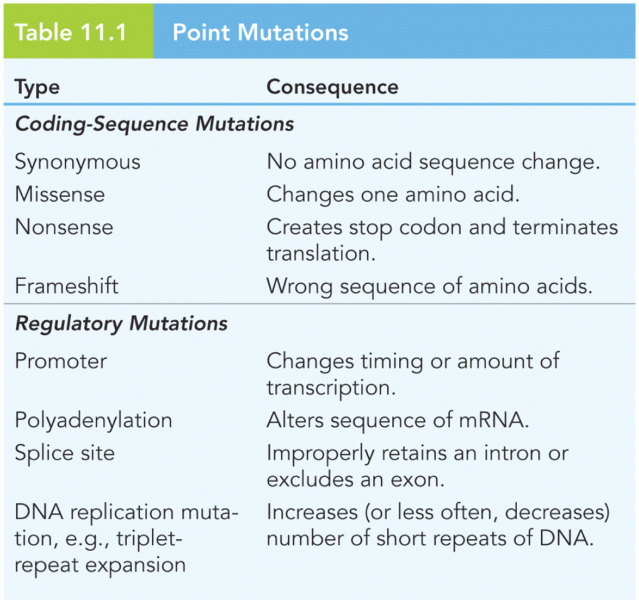
|
|
|
Multiple experimental evidences have confirmed that at the molecular level, cancer is caused by lesions in cellular DNA.
Vaccines cause herd immunity. If the majority of people in a community have been vaccinated against a disease, an unvaccinated person is less likely to get the disease since others are less likely to become sick from it and spread the disease.
All adults should have their cholesterol levels checked once every 5 years. During 2009–2010, 69.4% of Americans age 20 and older reported having their cholesterol checked within the last five years.
Many supplement containers do not even contain what their labels say. There are many documented reports of products containing much less, or more, that what is listed on their labels. They may also contain undisclosed prescription drugs and even contaminants.
It is widely believed that giving a daily oral dose of aspirin to heart attack patients improves their chances of survival because the aspirin blocks the formation of new blood clots.