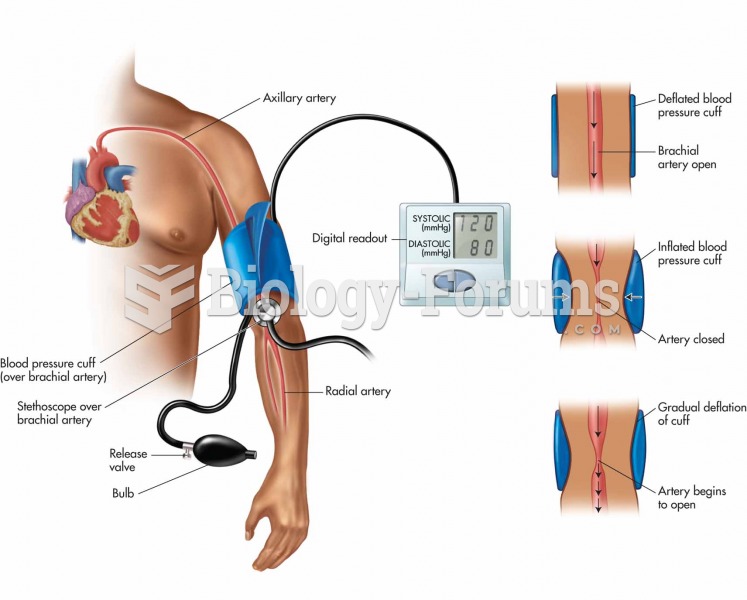
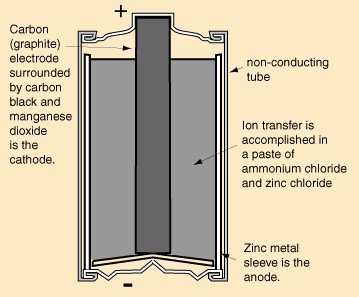
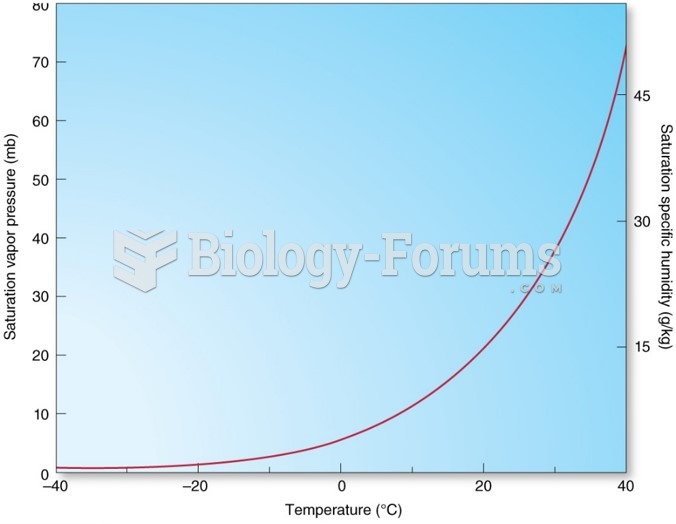
|
|
|
In 2012, nearly 24 milliion Americans, aged 12 and older, had abused an illicit drug, according to the National Institute on Drug Abuse (NIDA).
The human body produces and destroys 15 million blood cells every second.
Once thought to have neurofibromatosis, Joseph Merrick (also known as "the elephant man") is now, in retrospect, thought by clinical experts to have had Proteus syndrome. This endocrine disease causes continued and abnormal growth of the bones, muscles, skin, and so on and can become completely debilitating with severe deformities occurring anywhere on the body.
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.
In the United States, there is a birth every 8 seconds, according to the U.S. Census Bureau's Population Clock.