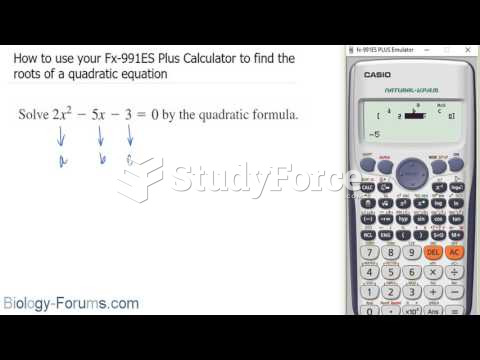
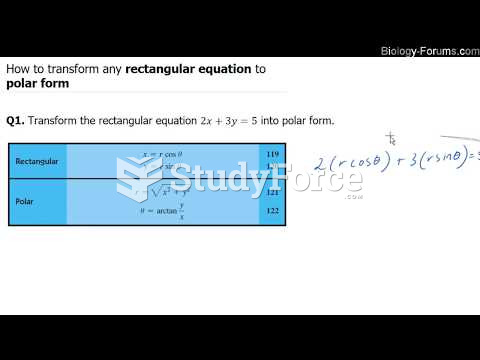
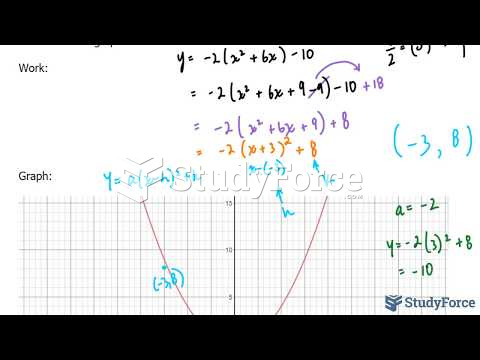
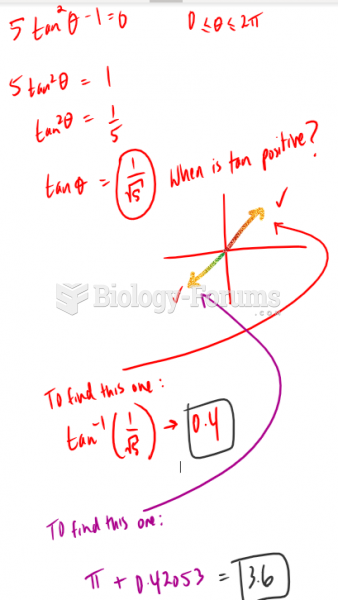
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The first monoclonal antibodies were made exclusively from mouse cells. Some are now fully human, which means they are likely to be safer and may be more effective than older monoclonal antibodies.
Did you know?
The lipid bilayer is made of phospholipids. They are arranged in a double layer because one of their ends is attracted to water while the other is repelled by water.
Did you know?
There are approximately 3 million unintended pregnancies in the United States each year.
Did you know?
Thyroid conditions may make getting pregnant impossible.
Did you know?
Many medications that are used to treat infertility are injected subcutaneously. This is easy to do using the anterior abdomen as the site of injection but avoiding the area directly around the belly button.