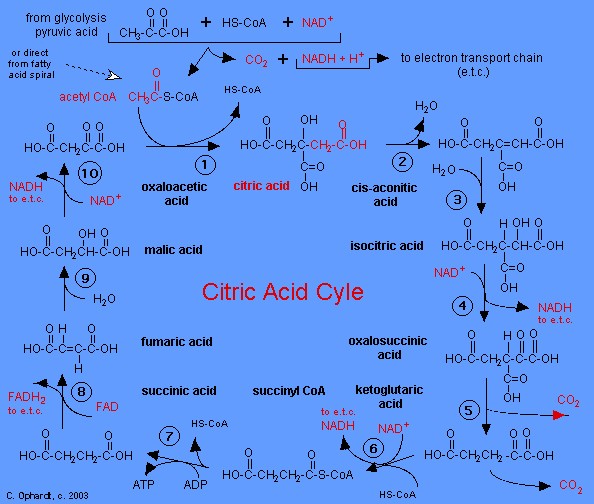
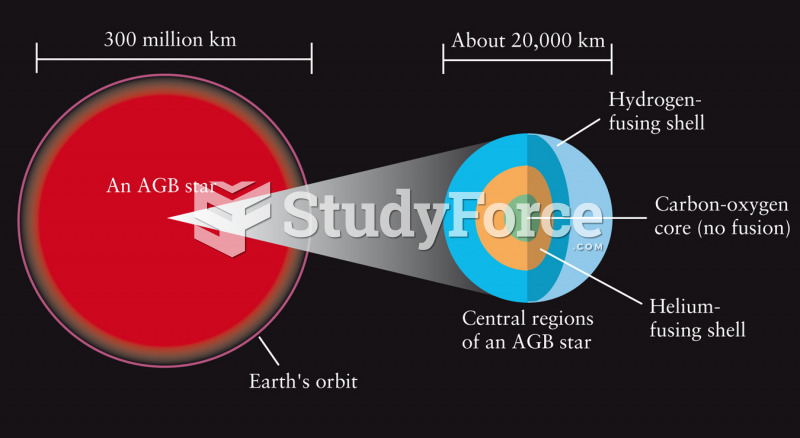
|
|
|
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.
More than 34,000 trademarked medication names and more than 10,000 generic medication names are in use in the United States.
In 1886, William Bates reported on the discovery of a substance produced by the adrenal gland that turned out to be epinephrine (adrenaline). In 1904, this drug was first artificially synthesized by Friedrich Stolz.
After 5 years of being diagnosed with rheumatoid arthritis, one every three patients will no longer be able to work.
There are immediate benefits of chiropractic adjustments that are visible via magnetic resonance imaging (MRI). It shows that spinal manipulation therapy is effective in decreasing pain and increasing the gaps between the vertebrae, reducing pressure that leads to pain.