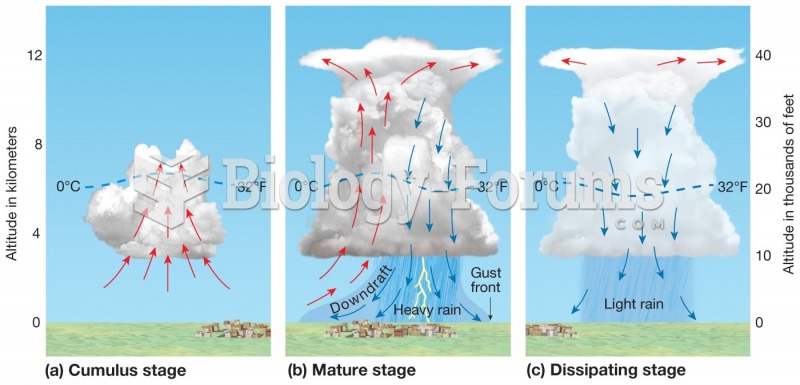
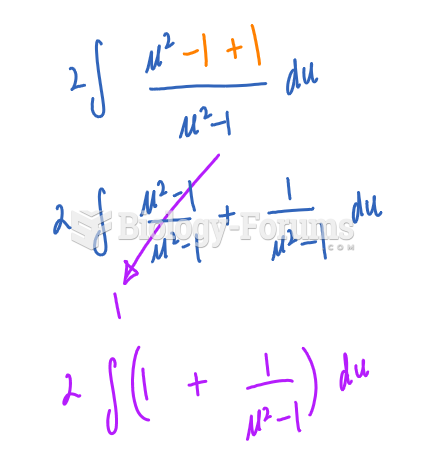|
|
|
In ancient Rome, many of the richer people in the population had lead-induced gout. The reason for this is unclear. Lead poisoning has also been linked to madness.
Anti-aging claims should not ever be believed. There is no supplement, medication, or any other substance that has been proven to slow or stop the aging process.
For high blood pressure (hypertension), a new class of drug, called a vasopeptidase blocker (inhibitor), has been developed. It decreases blood pressure by simultaneously dilating the peripheral arteries and increasing the body's loss of salt.
Approximately 500,000 babies are born each year in the United States to teenage mothers.
An identified risk factor for osteoporosis is the intake of excessive amounts of vitamin A. Dietary intake of approximately double the recommended daily amount of vitamin A, by women, has been shown to reduce bone mineral density and increase the chances for hip fractures compared with women who consumed the recommended daily amount (or less) of vitamin A.