|
|
|
In most climates, 8 to 10 glasses of water per day is recommended for adults. The best indicator for adequate fluid intake is frequent, clear urination.
Adolescents often feel clumsy during puberty because during this time of development, their hands and feet grow faster than their arms and legs do. The body is therefore out of proportion. One out of five adolescents actually experiences growing pains during this period.
No drugs are available to relieve parathyroid disease. Parathyroid disease is caused by a parathyroid tumor, and it needs to be removed by surgery.
About 80% of major fungal systemic infections are due to Candida albicans. Another form, Candida peritonitis, occurs most often in postoperative patients. A rare disease, Candida meningitis, may follow leukemia, kidney transplant, other immunosuppressed factors, or when suffering from Candida septicemia.
The types of cancer that alpha interferons are used to treat include hairy cell leukemia, melanoma, follicular non-Hodgkin's lymphoma, and AIDS-related Kaposi's sarcoma.
 The recent mass upheavals in Tunisia, Egypt, Libya, Yemen, and Syria gave political scientists a cha
The recent mass upheavals in Tunisia, Egypt, Libya, Yemen, and Syria gave political scientists a cha
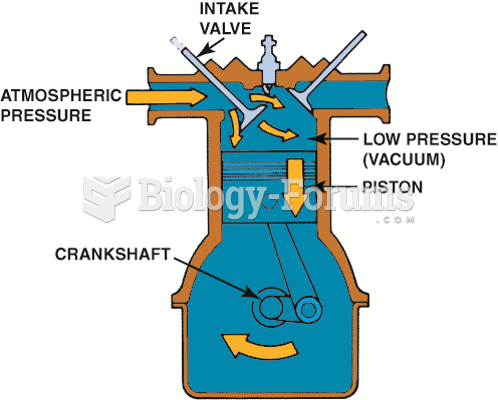 Downward movement of the piston lowers the air pressure inside the combustion chamber. The pressure ...
Downward movement of the piston lowers the air pressure inside the combustion chamber. The pressure ...