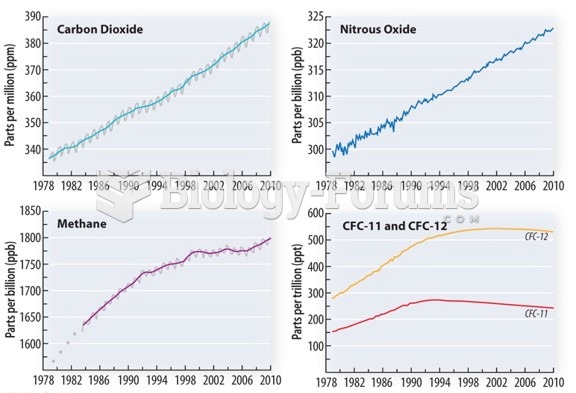
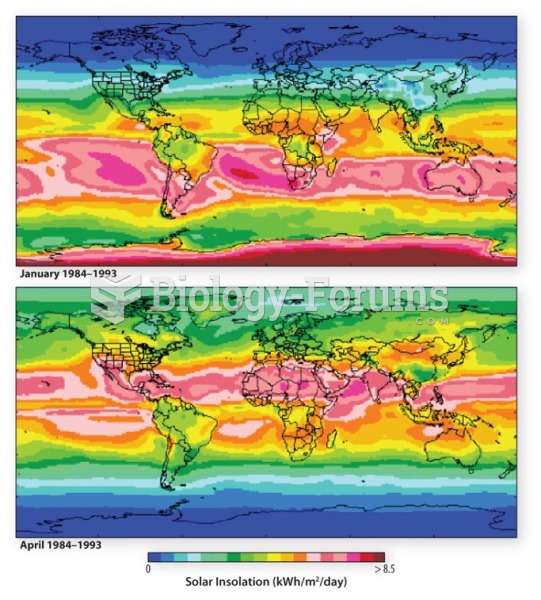
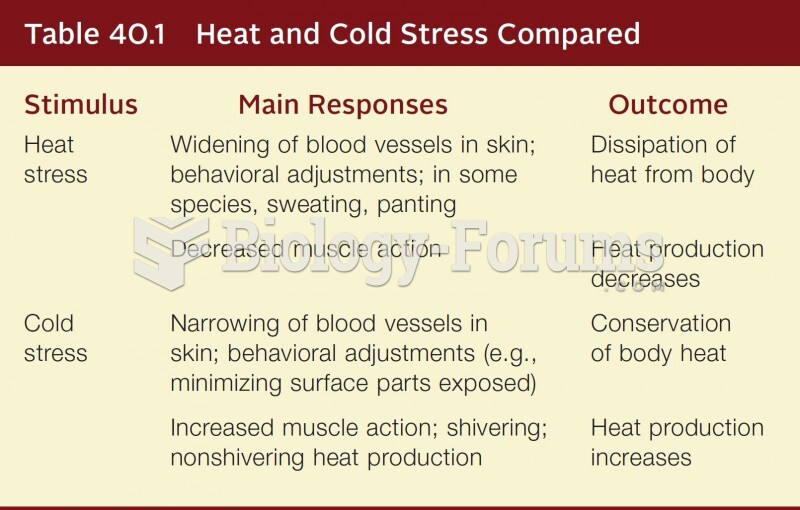
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
More than 150,000 Americans killed by cardiovascular disease are younger than the age of 65 years.
Did you know?
Approximately 500,000 babies are born each year in the United States to teenage mothers.
Did you know?
The National Institutes of Health have supported research into acupuncture. This has shown that acupuncture significantly reduced pain associated with osteoarthritis of the knee, when used as a complement to conventional therapies.
Did you know?
Women are two-thirds more likely than men to develop irritable bowel syndrome. This may be attributable to hormonal changes related to their menstrual cycles.
Did you know?
The term pharmacology is derived from the Greek words pharmakon("claim, medicine, poison, or remedy") and logos ("study").