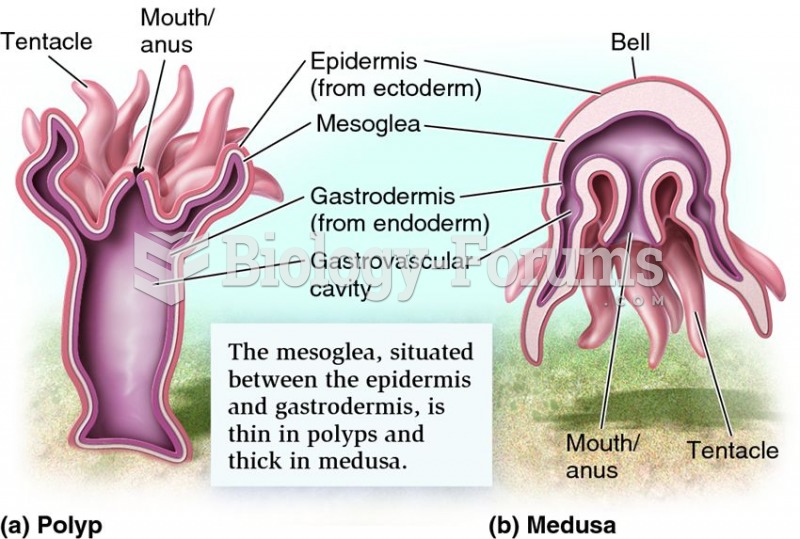
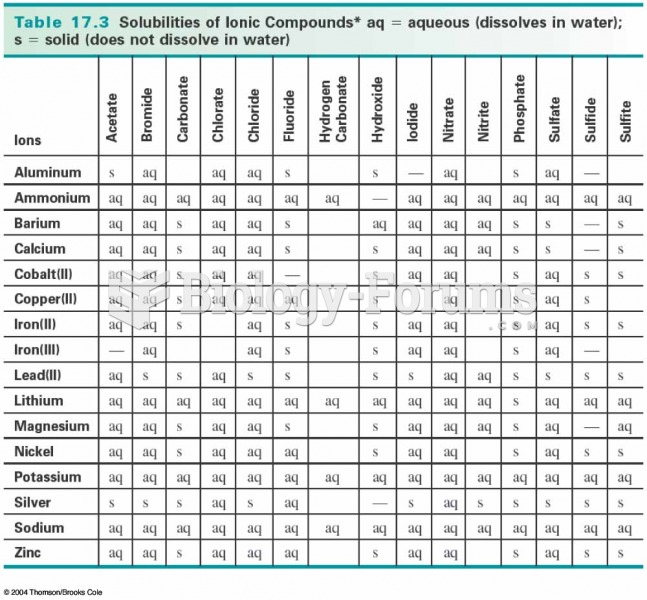
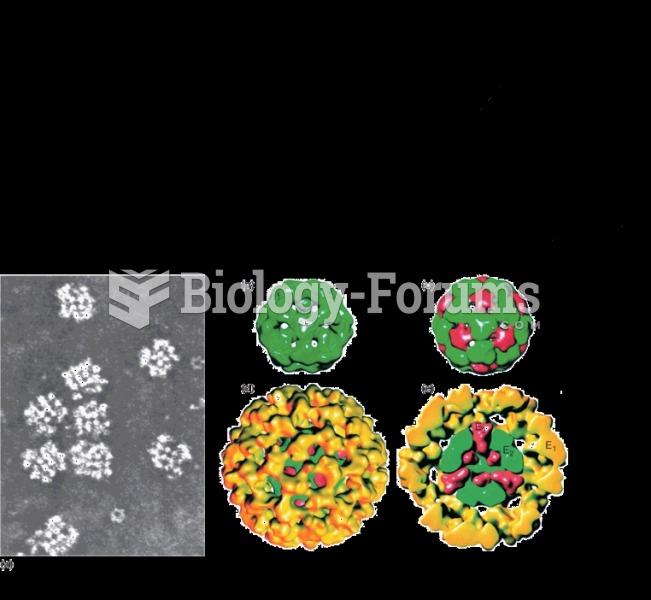
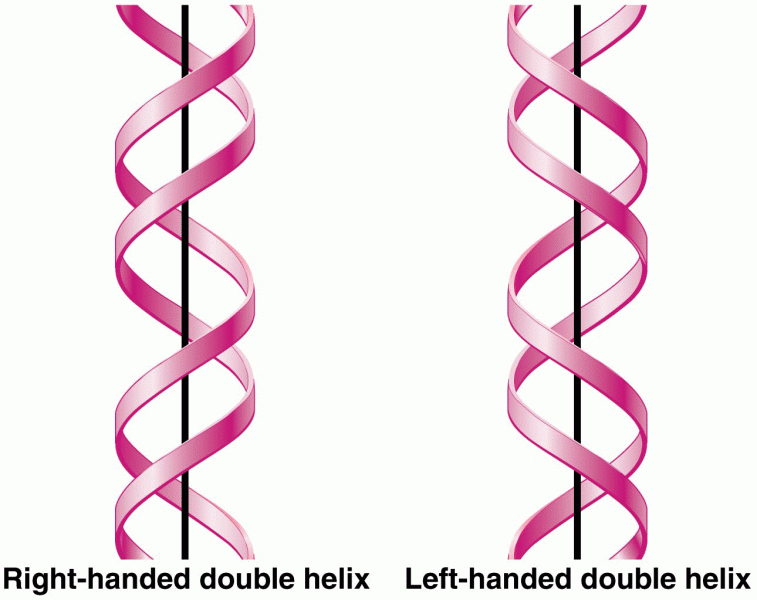
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Everyone has one nostril that is larger than the other.
Did you know?
The average adult has about 21 square feet of skin.
Did you know?
Parkinson's disease is both chronic and progressive. This means that it persists over a long period of time and that its symptoms grow worse over time.
Did you know?
Today, nearly 8 out of 10 pregnant women living with HIV (about 1.1 million), receive antiretrovirals.
Did you know?
Glaucoma is a leading cause of blindness. As of yet, there is no cure. Everyone is at risk, and there may be no warning signs. It is six to eight times more common in African Americans than in whites. The best and most effective way to detect glaucoma is to receive a dilated eye examination.