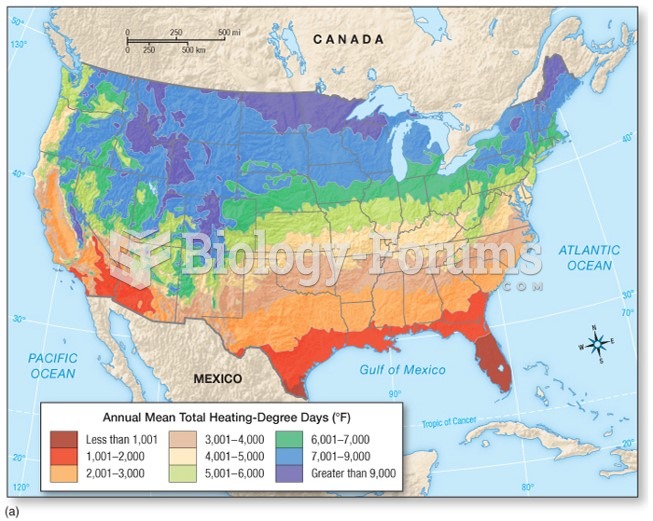
|
|
|
It is difficult to obtain enough calcium without consuming milk or other dairy foods.
The first monoclonal antibodies were made exclusively from mouse cells. Some are now fully human, which means they are likely to be safer and may be more effective than older monoclonal antibodies.
Methicillin-resistant Staphylococcus aureus or MRSA was discovered in 1961 in the United Kingdom. It if often referred to as a superbug. MRSA infections cause more deaths in the United States every year than AIDS.
Not getting enough sleep can greatly weaken the immune system. Lack of sleep makes you more likely to catch a cold, or more difficult to fight off an infection.
In the United States, there is a birth every 8 seconds, according to the U.S. Census Bureau's Population Clock.
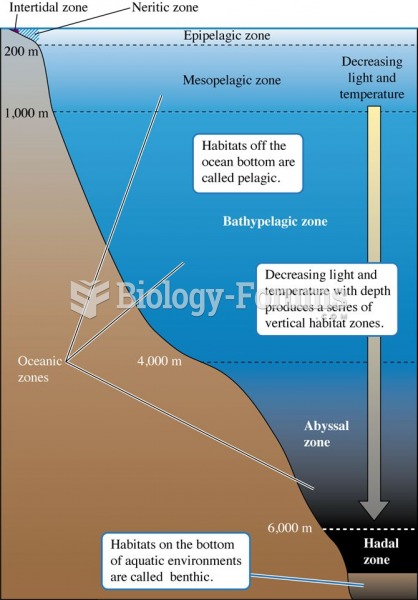 Vertical structuring of the oceans is associated with substantial variation in light and temperature
Vertical structuring of the oceans is associated with substantial variation in light and temperature
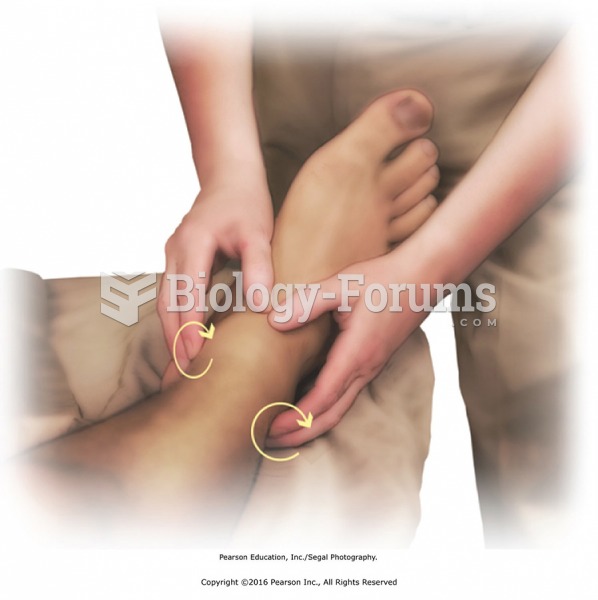
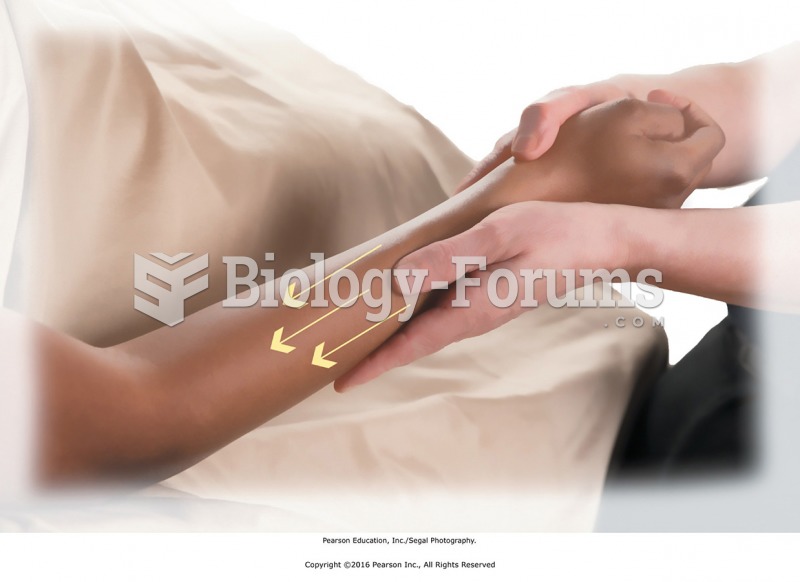 Stripping is performed with deep pressure along the length of a muscle to its attachment. Parallel ...
Stripping is performed with deep pressure along the length of a muscle to its attachment. Parallel ...