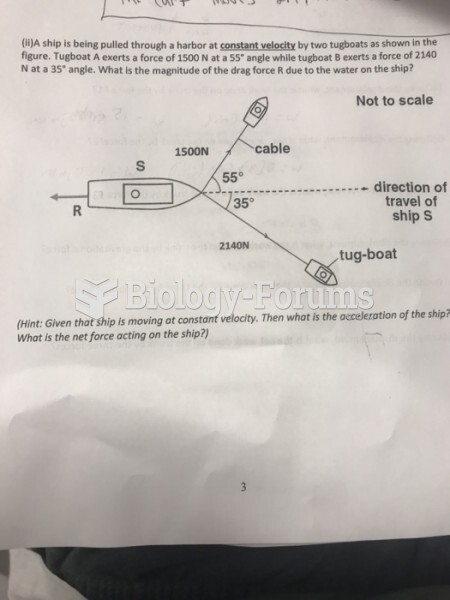
|
|
|
Did you know?
Increased intake of vitamin D has been shown to reduce fractures up to 25% in older people.
Did you know?
Each year in the United States, there are approximately six million pregnancies. This means that at any one time, about 4% of women in the United States are pregnant.
Did you know?
Methicillin-resistant Staphylococcus aureus or MRSA was discovered in 1961 in the United Kingdom. It if often referred to as a superbug. MRSA infections cause more deaths in the United States every year than AIDS.
Did you know?
In 1844, Charles Goodyear obtained the first patent for a rubber condom.
Did you know?
Approximately 70% of expectant mothers report experiencing some symptoms of morning sickness during the first trimester of pregnancy.