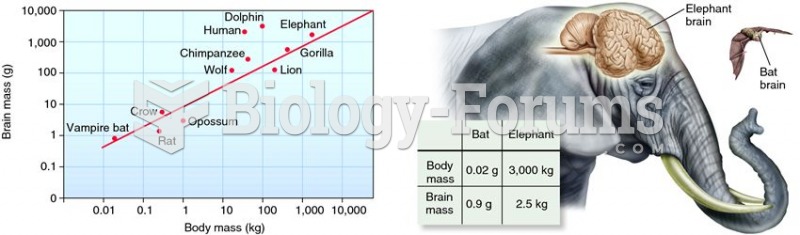
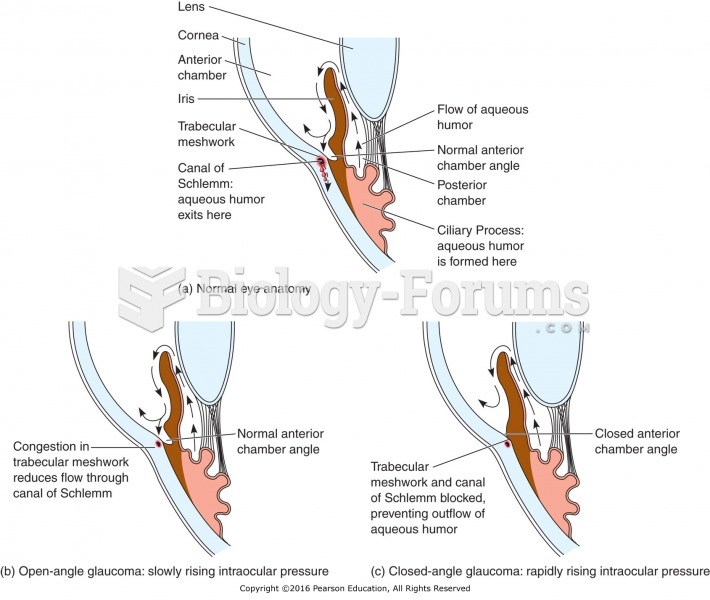
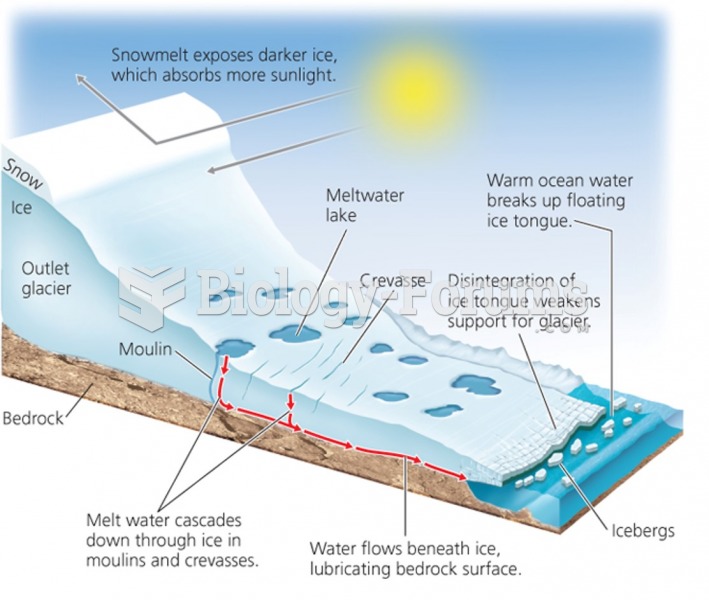
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The first documented use of surgical anesthesia in the United States was in Connecticut in 1844.
Did you know?
Each year in the United States, there are approximately six million pregnancies. This means that at any one time, about 4% of women in the United States are pregnant.
Did you know?
On average, the stomach produces 2 L of hydrochloric acid per day.
Did you know?
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.
Did you know?
Medication errors are three times higher among children and infants than with adults.