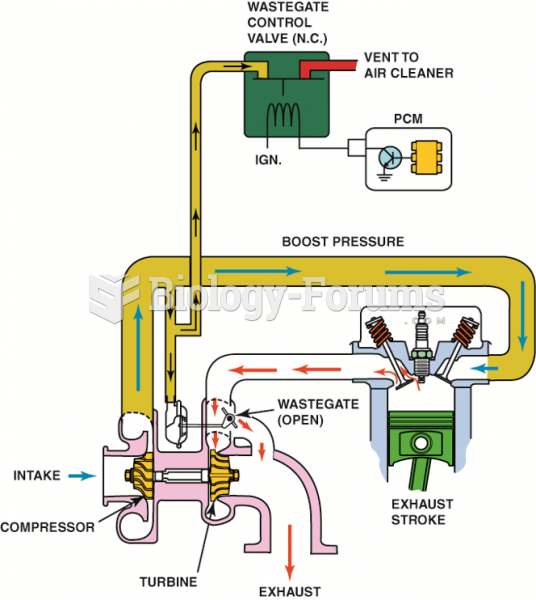
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The first documented use of surgical anesthesia in the United States was in Connecticut in 1844.
Did you know?
The first war in which wide-scale use of anesthetics occurred was the Civil War, and 80% of all wounds were in the extremities.
Did you know?
Glaucoma is a leading cause of blindness. As of yet, there is no cure. Everyone is at risk, and there may be no warning signs. It is six to eight times more common in African Americans than in whites. The best and most effective way to detect glaucoma is to receive a dilated eye examination.
Did you know?
Human kidneys will clean about 1 million gallons of blood in an average lifetime.
Did you know?
It is difficult to obtain enough calcium without consuming milk or other dairy foods.