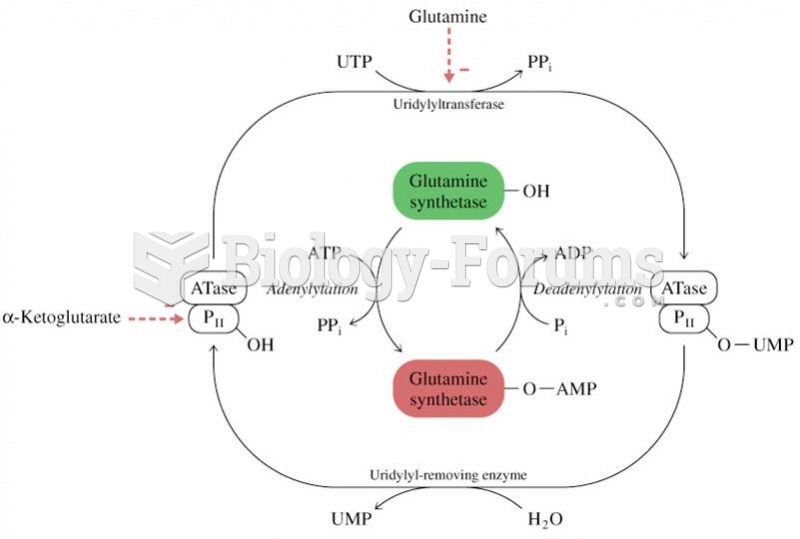
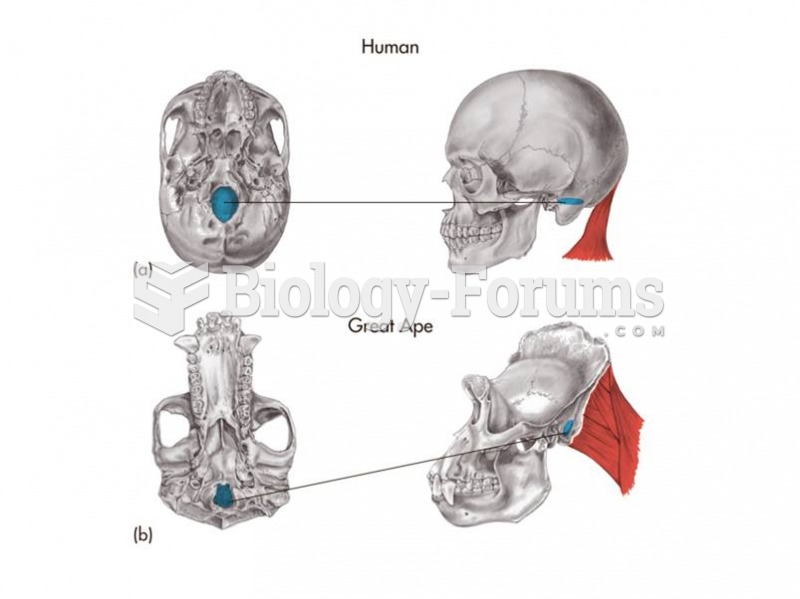
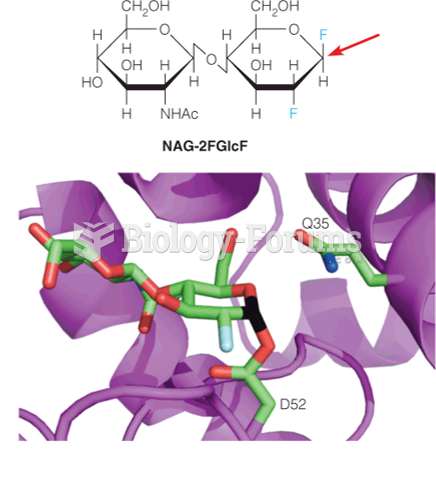
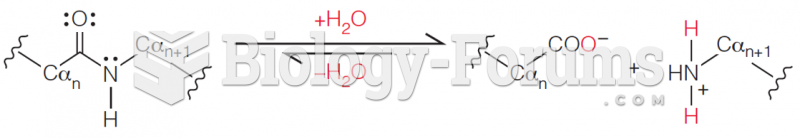|
|
|
Did you know?
Since 1988, the CDC has reported a 99% reduction in bacterial meningitis caused by Haemophilus influenzae, due to the introduction of the vaccine against it.
Did you know?
Increased intake of vitamin D has been shown to reduce fractures up to 25% in older people.
Did you know?
The most destructive flu epidemic of all times in recorded history occurred in 1918, with approximately 20 million deaths worldwide.
Did you know?
Between 1999 and 2012, American adults with high total cholesterol decreased from 18.3% to 12.9%
Did you know?
The first war in which wide-scale use of anesthetics occurred was the Civil War, and 80% of all wounds were in the extremities.