|
|
|
Earwax has antimicrobial properties that reduce the viability of bacteria and fungus in the human ear.
Only one in 10 cancer deaths is caused by the primary tumor. The vast majority of cancer mortality is caused by cells breaking away from the main tumor and metastasizing to other parts of the body, such as the brain, bones, or liver.
In 2006, a generic antinausea drug named ondansetron was approved. It is used to stop nausea and vomiting associated with surgery, chemotherapy, and radiation therapy.
In the United States, an estimated 50 million unnecessary antibiotics are prescribed for viral respiratory infections.
The modern decimal position system was the invention of the Hindus (around 800 AD), involving the placing of numerals to indicate their value (units, tens, hundreds, and so on).
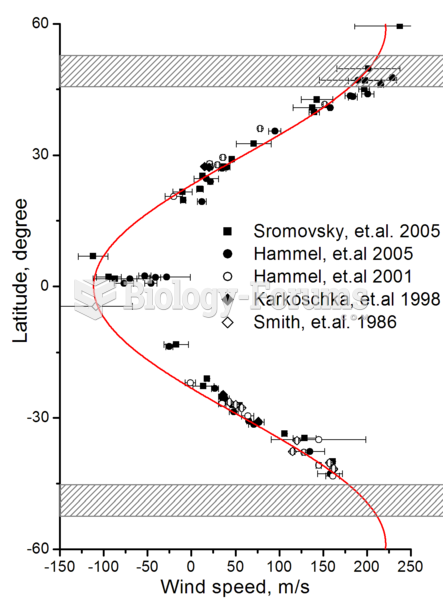 Zonal wind speeds on Uranus. Shaded areas show the southern collar and its future northern counterpa
Zonal wind speeds on Uranus. Shaded areas show the southern collar and its future northern counterpa
 The tarsier is a haplorhine, and may represent an evolutionary bridge between lower and higher prima
The tarsier is a haplorhine, and may represent an evolutionary bridge between lower and higher prima