This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
There are approximately 3 million unintended pregnancies in the United States each year.
Did you know?
As of mid-2016, 18.2 million people were receiving advanced retroviral therapy (ART) worldwide. This represents between 43–50% of the 34–39.8 million people living with HIV.
Did you know?
Fungal nail infections account for up to 30% of all skin infections. They affect 5% of the general population—mostly people over the age of 70.
Did you know?
Autoimmune diseases occur when the immune system destroys its own healthy tissues. When this occurs, white blood cells cannot distinguish between pathogens and normal cells.
Did you know?
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.
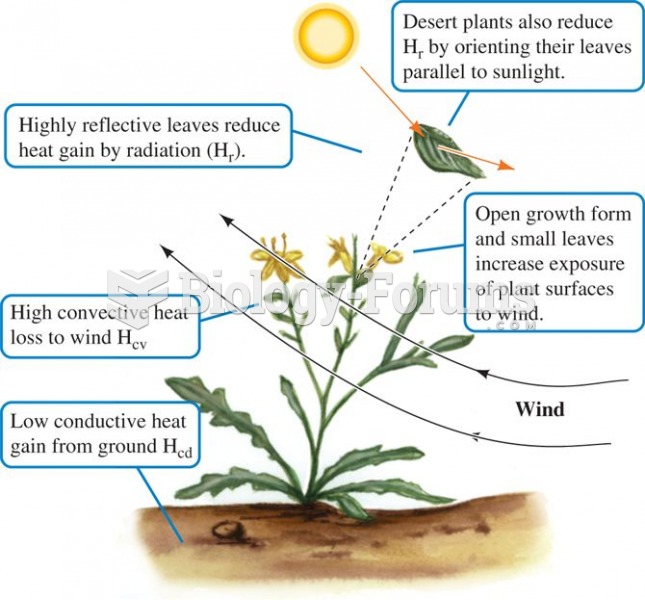 The form and orientation of desert plants reduces heat gain from the environment and facilitates coo
The form and orientation of desert plants reduces heat gain from the environment and facilitates coo
 The patient is undergoing an allergy skin test by receiving subdermal inoculations of allergens. Inf
The patient is undergoing an allergy skin test by receiving subdermal inoculations of allergens. Inf
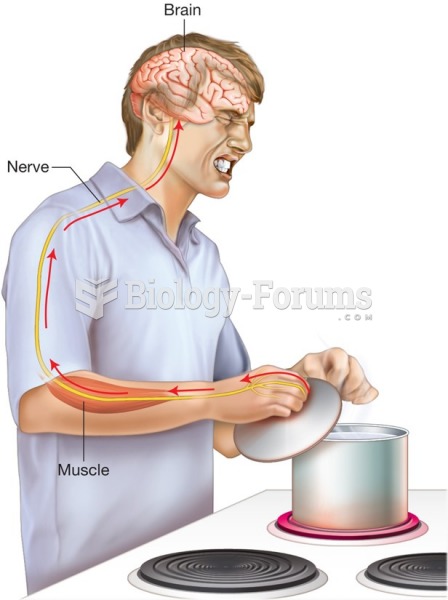 The integumentary and nervous systems work together to recognize specific sensations, such as heat ...
The integumentary and nervous systems work together to recognize specific sensations, such as heat ...