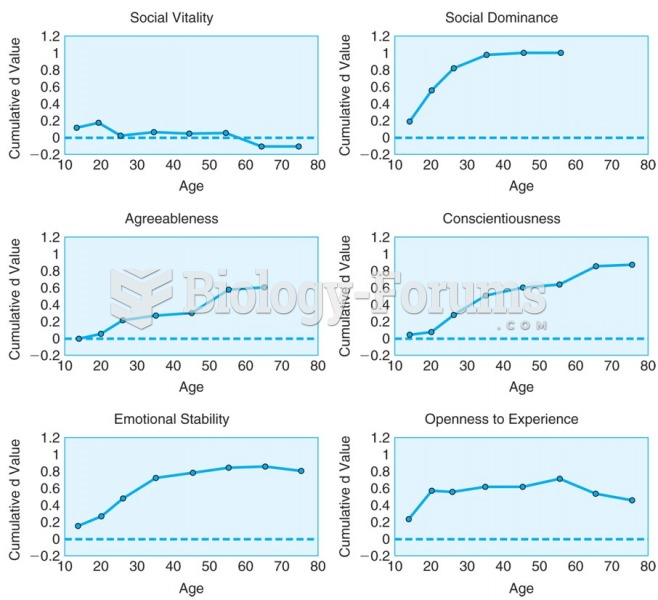
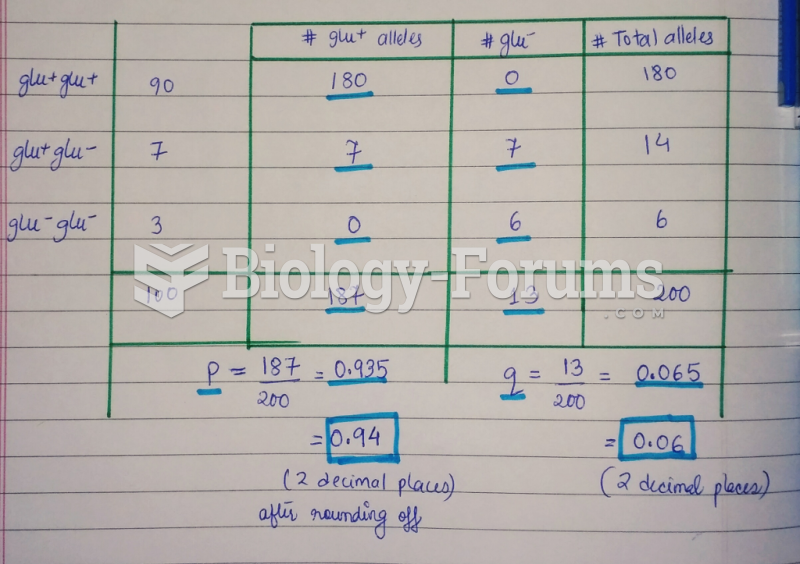
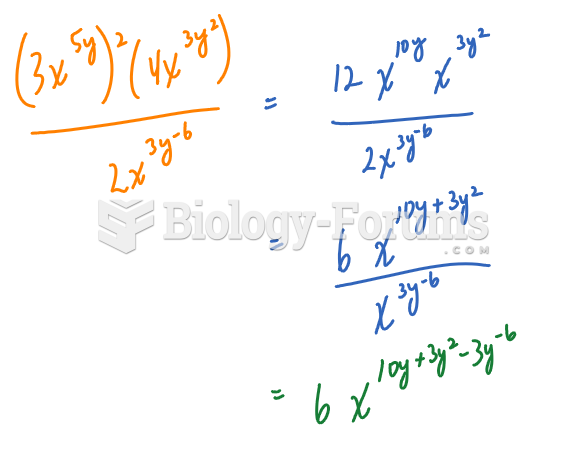This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The immune system needs 9.5 hours of sleep in total darkness to recharge completely.
Did you know?
Serum cholesterol testing in adults is recommended every 1 to 5 years. People with diabetes and a family history of high cholesterol should be tested even more frequently.
Did you know?
Women are two-thirds more likely than men to develop irritable bowel syndrome. This may be attributable to hormonal changes related to their menstrual cycles.
Did you know?
Blood in the urine can be a sign of a kidney stone, glomerulonephritis, or other kidney problems.
Did you know?
In 1864, the first barbiturate (barbituric acid) was synthesized.