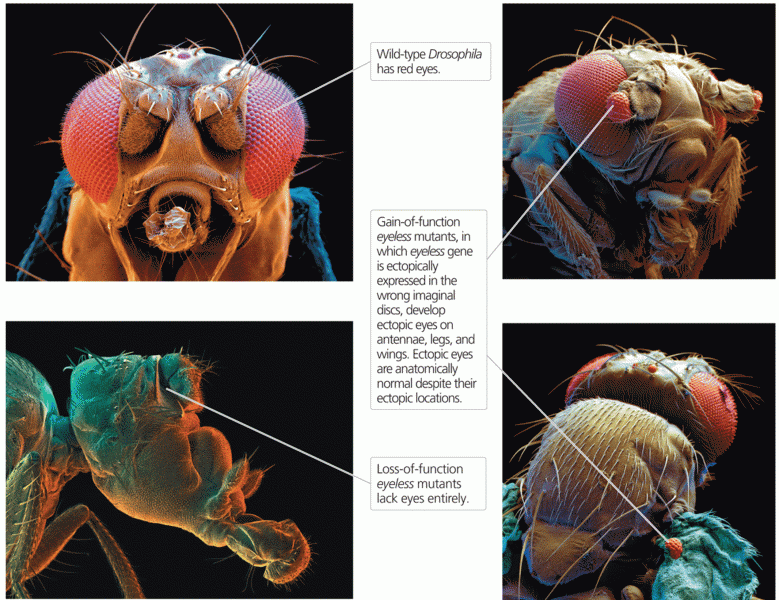
|
|
|
The largest baby ever born weighed more than 23 pounds but died just 11 hours after his birth in 1879. The largest surviving baby was born in October 2009 in Sumatra, Indonesia, and weighed an astounding 19.2 pounds at birth.
Glaucoma is a leading cause of blindness. As of yet, there is no cure. Everyone is at risk, and there may be no warning signs. It is six to eight times more common in African Americans than in whites. The best and most effective way to detect glaucoma is to receive a dilated eye examination.
In 1844, Charles Goodyear obtained the first patent for a rubber condom.
Despite claims by manufacturers, the supplement known as Ginkgo biloba was shown in a study of more than 3,000 participants to be ineffective in reducing development of dementia and Alzheimer’s disease in older people.
In Eastern Europe and Russia, interferon is administered intranasally in varied doses for the common cold and influenza. It is claimed that this treatment can lower the risk of infection by as much as 60–70%.
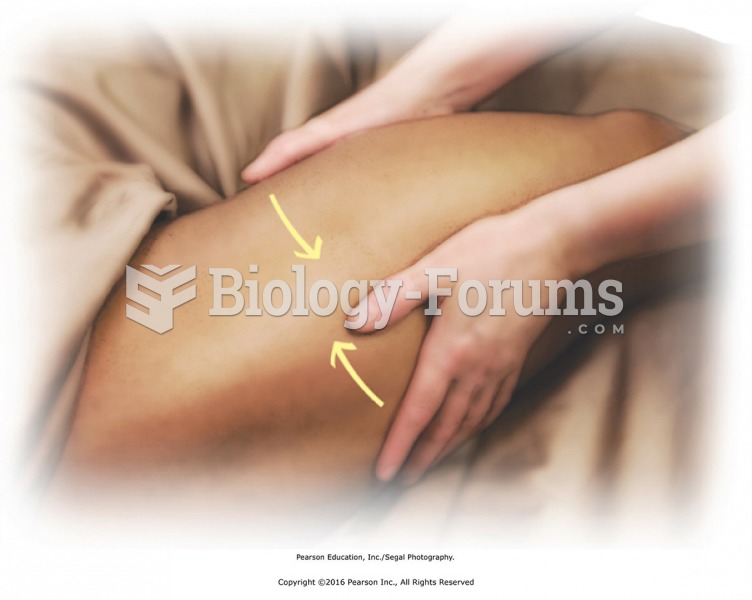 Jostle the thigh, tossing from side to side, hand to hand. Causes loose, free movement in the hip ...
Jostle the thigh, tossing from side to side, hand to hand. Causes loose, free movement in the hip ...
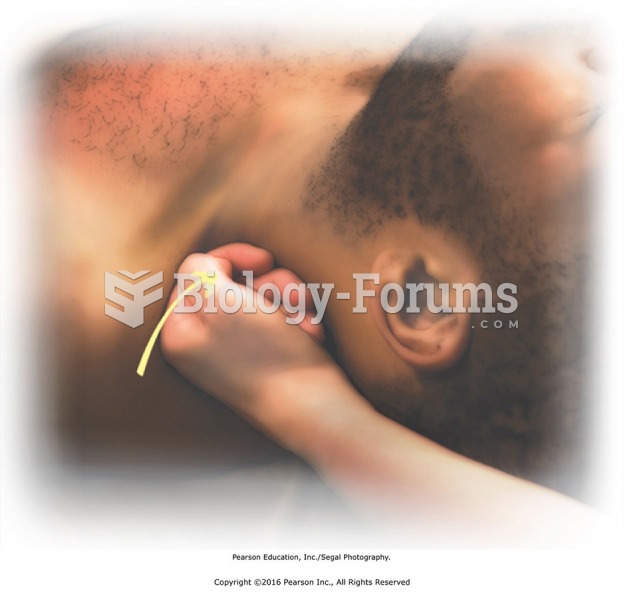 Effleurage with loose fist to left side of neck. Turn the head slightly to the right side, exposing ...
Effleurage with loose fist to left side of neck. Turn the head slightly to the right side, exposing ...