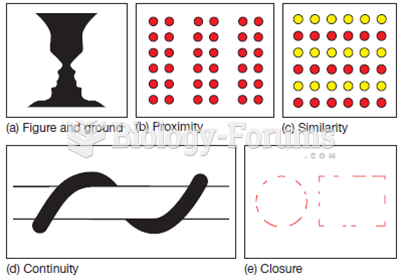
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Limit intake of red meat and dairy products made with whole milk. Choose skim milk, low-fat or fat-free dairy products. Limit fried food. Use healthy oils when cooking.
Did you know?
Thyroid conditions may make getting pregnant impossible.
Did you know?
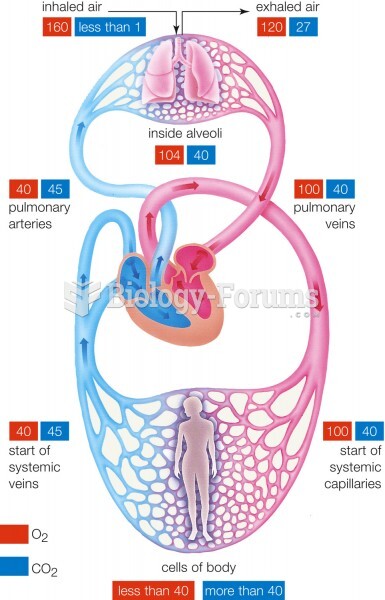
There are 60,000 miles of blood vessels in every adult human.
Did you know?
Vital signs (blood pressure, temperature, pulse rate, respiration rate) should be taken before any drug administration. Patients should be informed not to use tobacco or caffeine at least 30 minutes before their appointment.
Did you know?
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.