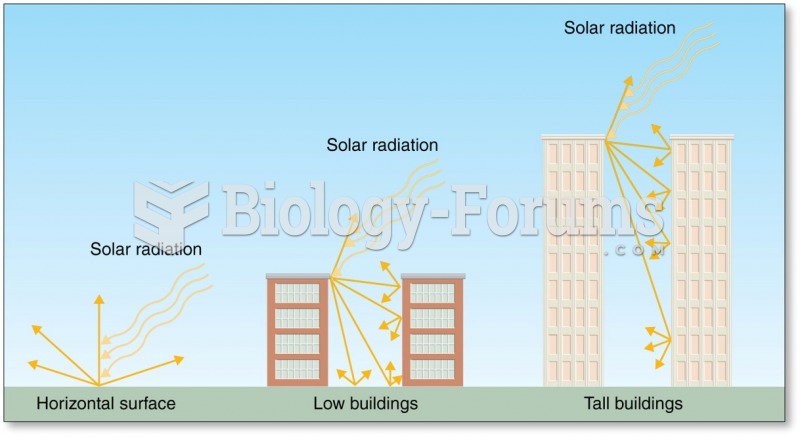
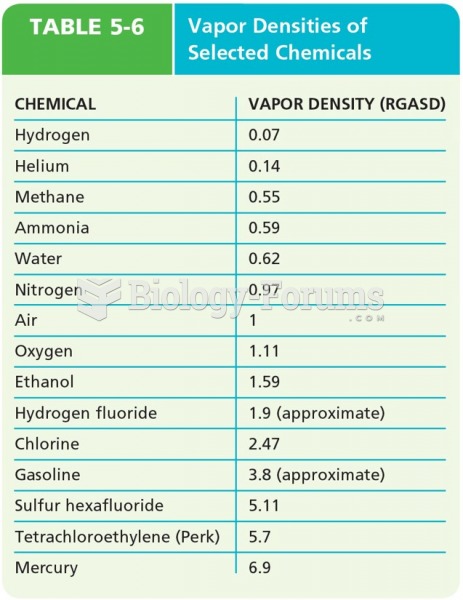
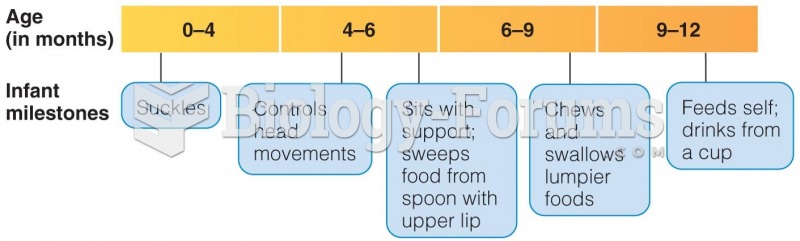
|
|
|
During the twentieth century, a variant of the metric system was used in Russia and France in which the base unit of mass was the tonne. Instead of kilograms, this system used millitonnes (mt).
HIV testing reach is still limited. An estimated 40% of people with HIV (more than 14 million) remain undiagnosed and do not know their infection status.
Many of the drugs used by neuroscientists are derived from toxic plants and venomous animals (such as snakes, spiders, snails, and puffer fish).
Your skin wrinkles if you stay in the bathtub a long time because the outermost layer of skin (which consists of dead keratin) swells when it absorbs water. It is tightly attached to the skin below it, so it compensates for the increased area by wrinkling. This happens to the hands and feet because they have the thickest layer of dead keratin cells.
Approximately 500,000 babies are born each year in the United States to teenage mothers.
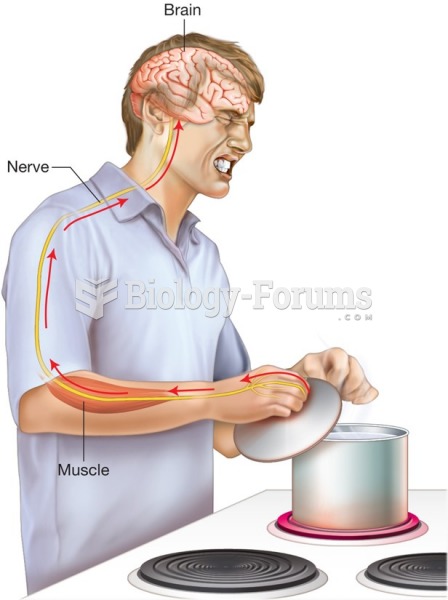 The integumentary and nervous systems work together to recognize specific sensations, such as heat ...
The integumentary and nervous systems work together to recognize specific sensations, such as heat ...
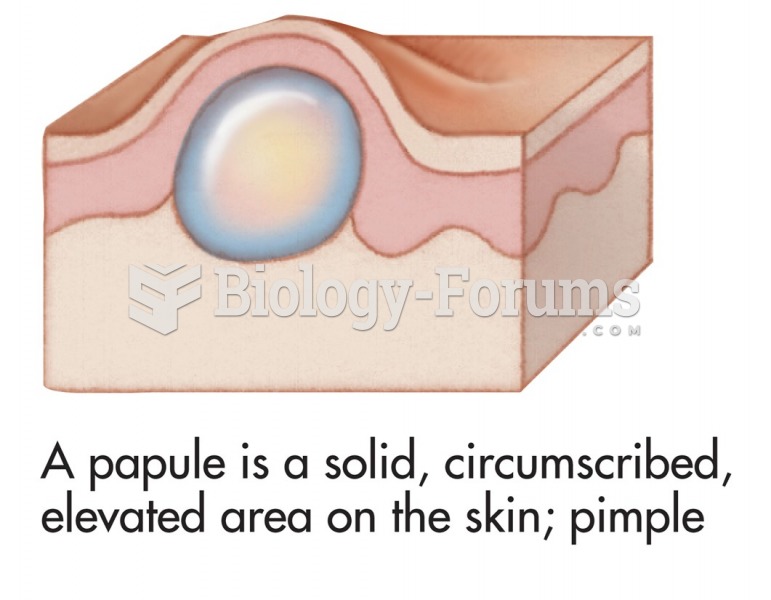 Common skin signs are often evidence of an illness or disorder. A papule is a solid, circumscribed, ...
Common skin signs are often evidence of an illness or disorder. A papule is a solid, circumscribed, ...