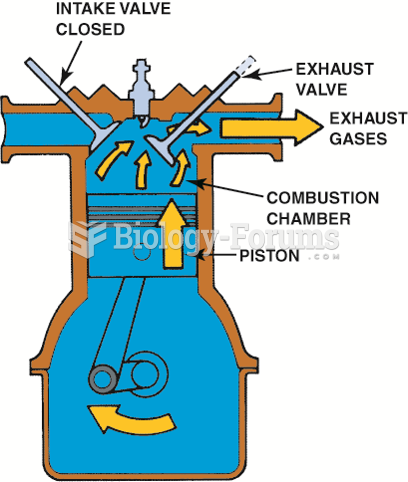
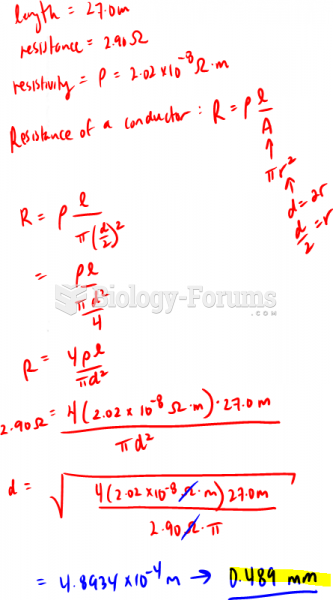
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Signs and symptoms of a drug overdose include losing consciousness, fever or sweating, breathing problems, abnormal pulse, and changes in skin color.
Did you know?
Symptoms of kidney problems include a loss of appetite, back pain (which may be sudden and intense), chills, abdominal pain, fluid retention, nausea, the urge to urinate, vomiting, and fever.
Did you know?
In 1864, the first barbiturate (barbituric acid) was synthesized.
Did you know?
Pubic lice (crabs) are usually spread through sexual contact. You cannot catch them by using a public toilet.
Did you know?
The Babylonians wrote numbers in a system that used 60 as the base value rather than the number 10. They did not have a symbol for "zero."