|
|
|
In Eastern Europe and Russia, interferon is administered intranasally in varied doses for the common cold and influenza. It is claimed that this treatment can lower the risk of infection by as much as 60–70%.
Pregnant women usually experience a heightened sense of smell beginning late in the first trimester. Some experts call this the body's way of protecting a pregnant woman from foods that are unsafe for the fetus.
People with alcoholism are at a much greater risk of malnutrition than are other people and usually exhibit low levels of most vitamins (especially folic acid). This is because alcohol often takes the place of 50% of their daily intake of calories, with little nutritional value contained in it.
A recent study has found that following a diet rich in berries may slow down the aging process of the brain. This diet apparently helps to keep dopamine levels much higher than are seen in normal individuals who do not eat berries as a regular part of their diet as they enter their later years.
People about to have surgery must tell their health care providers about all supplements they take.
 The recent mass upheavals in Tunisia, Egypt, Libya, Yemen, and Syria gave political scientists a cha
The recent mass upheavals in Tunisia, Egypt, Libya, Yemen, and Syria gave political scientists a cha
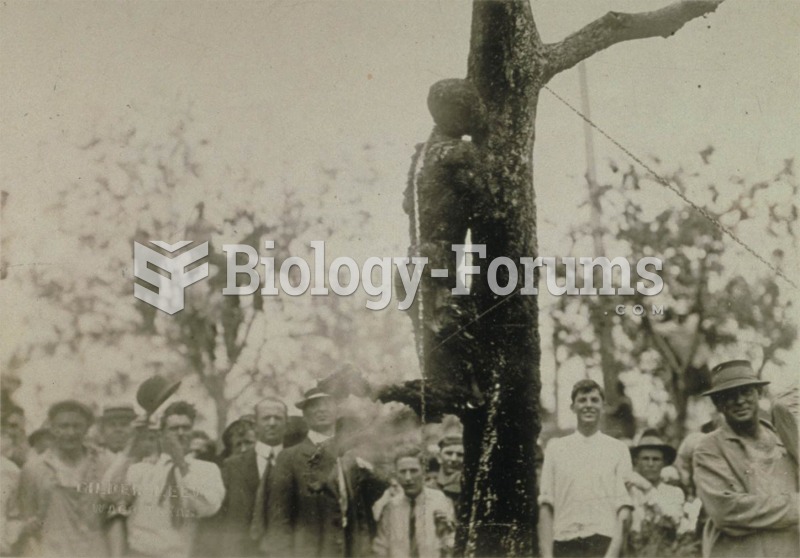 On May 15, 1916, after deliberating for one hour, an all-white jury in Waco, Texas, found seventeen-
On May 15, 1916, after deliberating for one hour, an all-white jury in Waco, Texas, found seventeen-