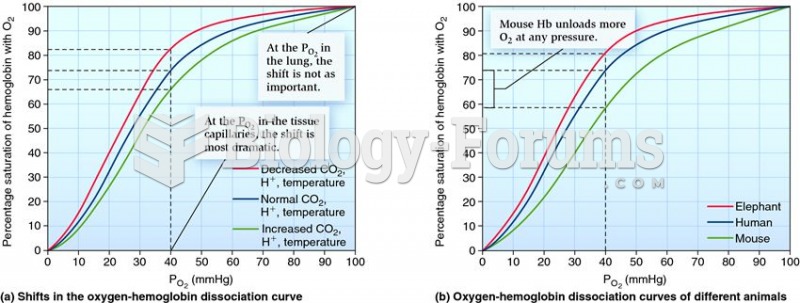
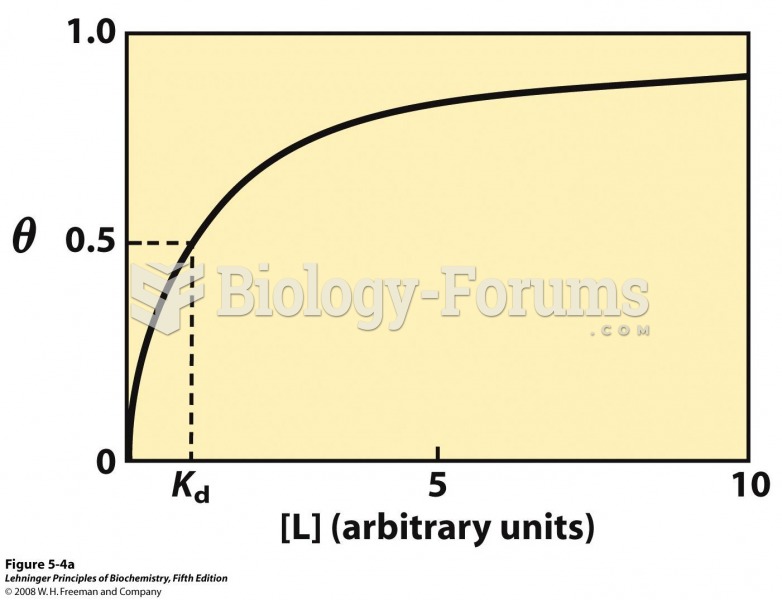
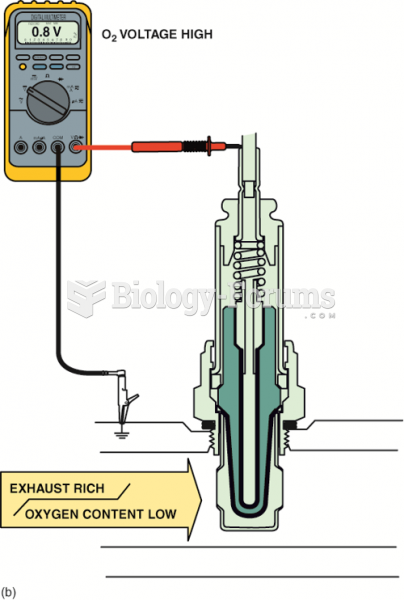
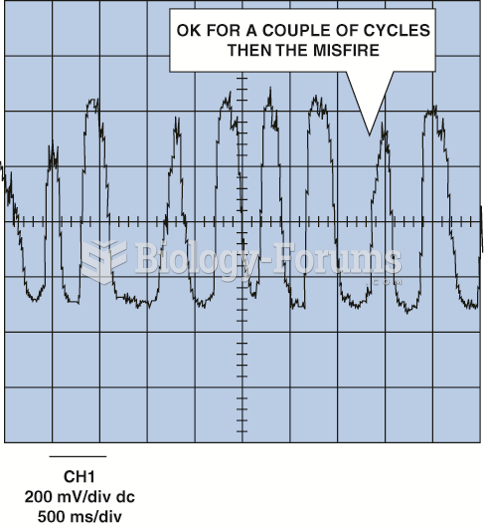
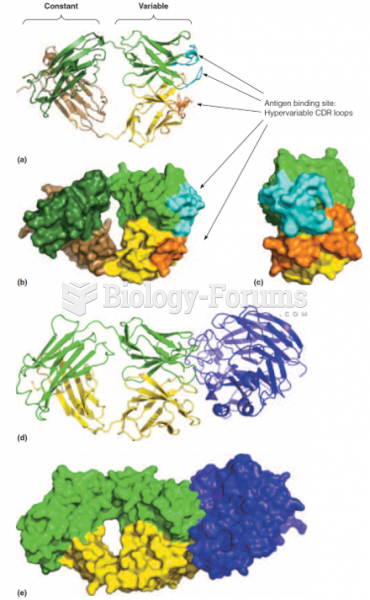
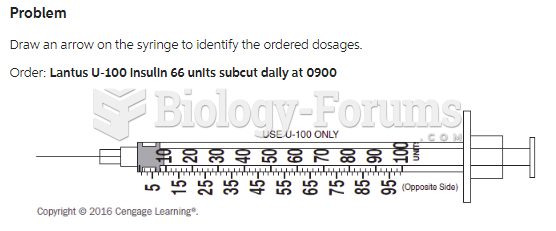
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
If all the neurons in the human body were lined up, they would stretch more than 600 miles.
Did you know?
Your chance of developing a kidney stone is 1 in 10. In recent years, approximately 3.7 million people in the United States were diagnosed with a kidney disease.
Did you know?
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.
Did you know?
There are over 65,000 known species of protozoa. About 10,000 species are parasitic.
Did you know?
Normal urine is sterile. It contains fluids, salts, and waste products. It is free of bacteria, viruses, and fungi.