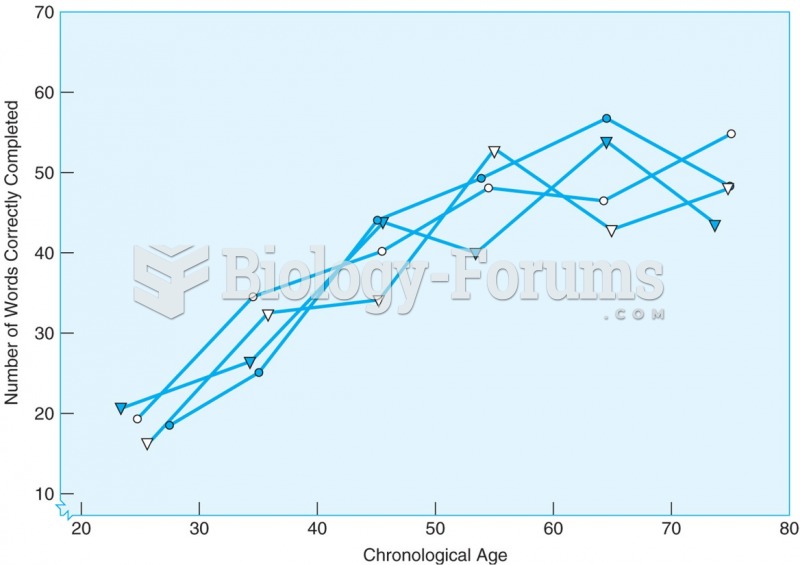
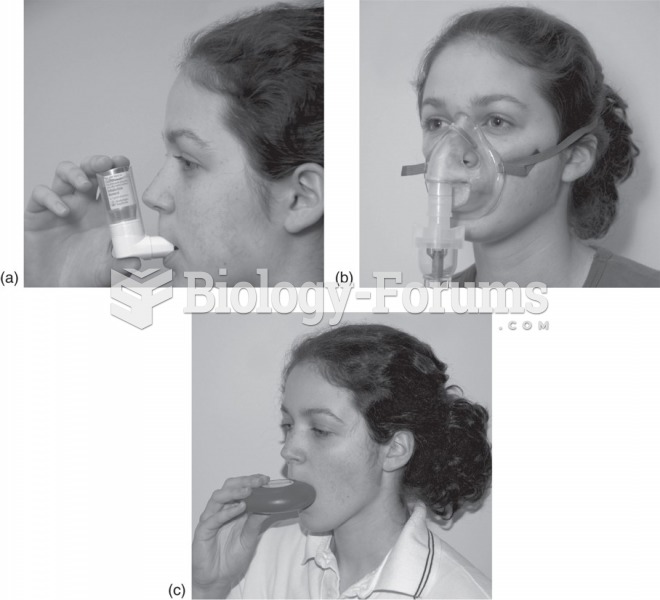
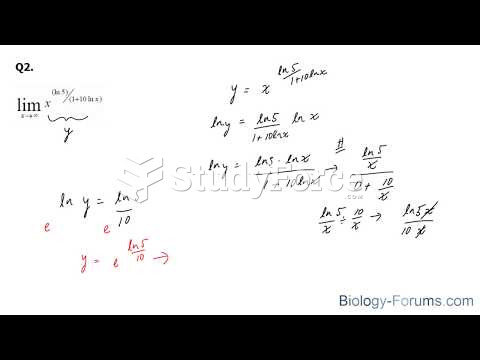
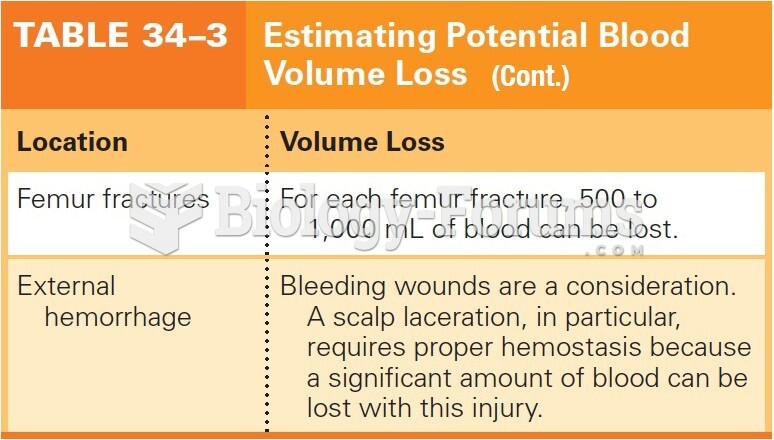
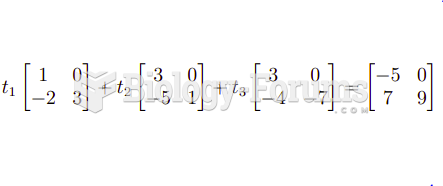|
|
|
If all the neurons in the human body were lined up, they would stretch more than 600 miles.
Automated pill dispensing systems have alarms to alert patients when the correct dosing time has arrived. Most systems work with many varieties of medications, so patients who are taking a variety of drugs can still be in control of their dose regimen.
When intravenous medications are involved in adverse drug events, their harmful effects may occur more rapidly, and be more severe than errors with oral medications. This is due to the direct administration into the bloodstream.
The first oncogene was discovered in 1970 and was termed SRC (pronounced "SARK").
More than 150,000 Americans killed by cardiovascular disease are younger than the age of 65 years.