|
|
|
According to the Migraine Research Foundation, migraines are the third most prevalent illness in the world. Women are most affected (18%), followed by children of both sexes (10%), and men (6%).
There are more sensory neurons in the tongue than in any other part of the body.
About one in five American adults and teenagers have had a genital herpes infection—and most of them don't know it. People with genital herpes have at least twice the risk of becoming infected with HIV if exposed to it than those people who do not have genital herpes.
Signs of depression include feeling sad most of the time for 2 weeks or longer; loss of interest in things normally enjoyed; lack of energy; sleep and appetite disturbances; weight changes; feelings of hopelessness, helplessness, or worthlessness; an inability to make decisions; and thoughts of death and suicide.
Children with strabismus (crossed eyes) can be treated. They are not able to outgrow this condition on their own, but with help, it can be more easily corrected at a younger age. It is important for infants to have eye examinations as early as possible in their development and then another at age 2 years.
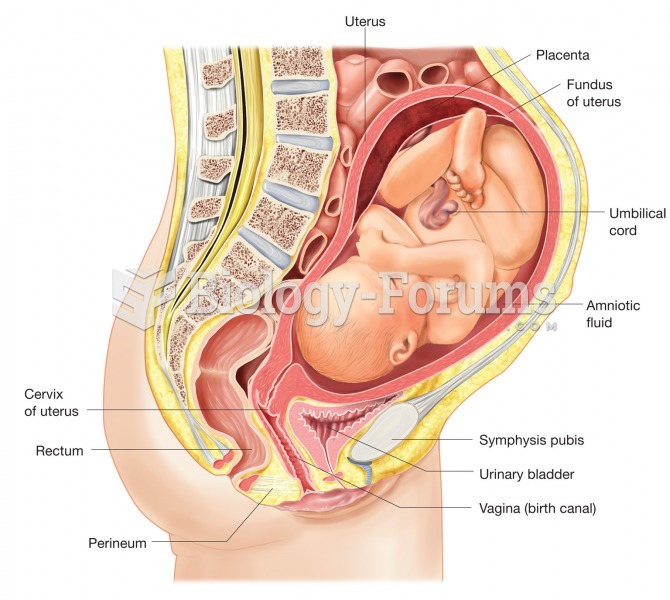 A full-term pregnancy. Image illustrates position of the fetus and the structures associated with pr
A full-term pregnancy. Image illustrates position of the fetus and the structures associated with pr
 Erythroblastosis fetalis. (a) The condition occurs with an RH+ father and RH– mother. (b) First preg
Erythroblastosis fetalis. (a) The condition occurs with an RH+ father and RH– mother. (b) First preg





