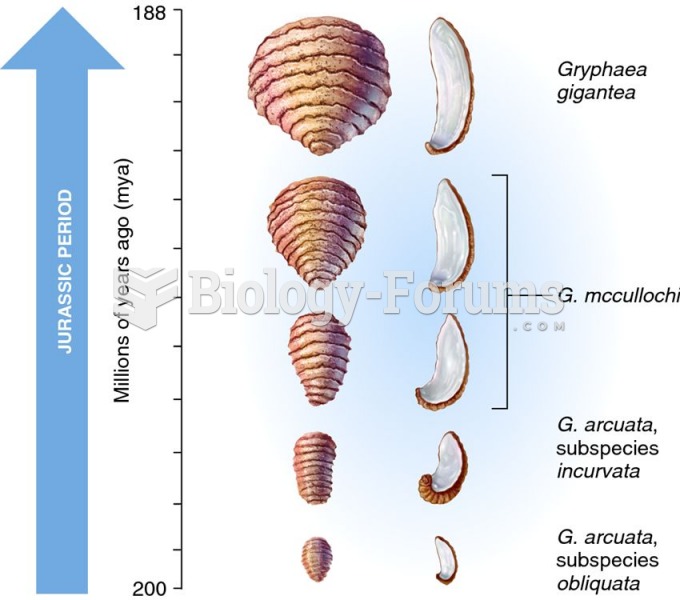
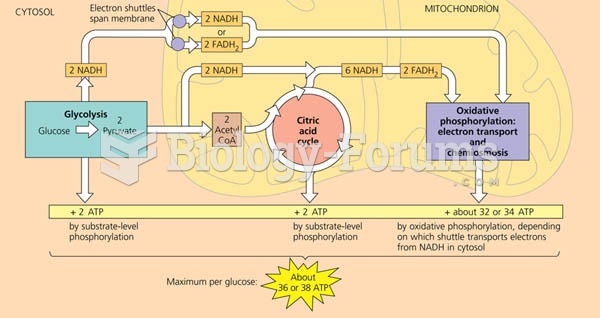
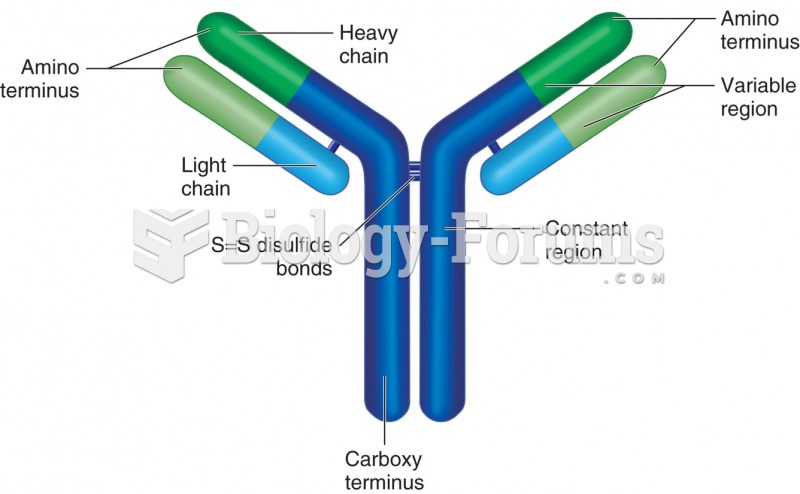
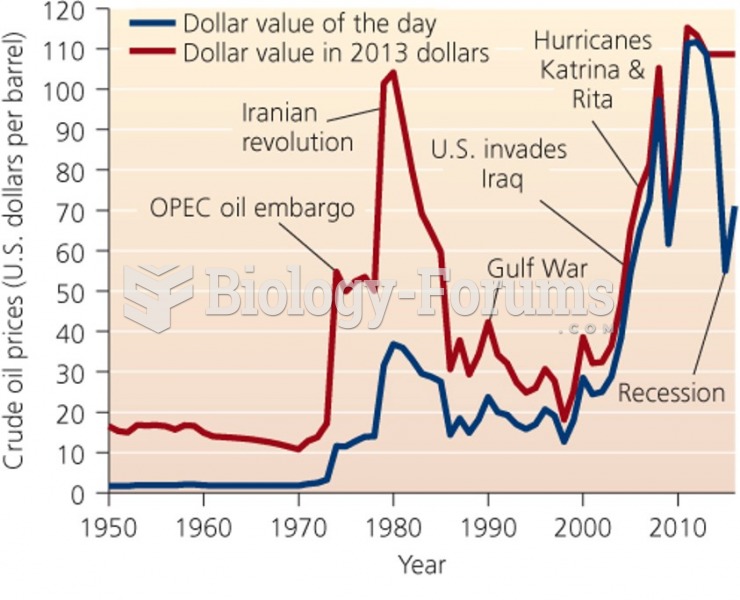
|
|
|
Medications that are definitely not safe to take when breastfeeding include radioactive drugs, antimetabolites, some cancer (chemotherapy) agents, bromocriptine, ergotamine, methotrexate, and cyclosporine.
Disorders that may affect pharmacodynamics include genetic mutations, malnutrition, thyrotoxicosis, myasthenia gravis, Parkinson's disease, and certain forms of insulin-resistant diabetes mellitus.
The U.S. Preventive Services Task Force recommends that all women age 65 years of age or older should be screened with bone densitometry.
According to the FDA, adverse drug events harmed or killed approximately 1,200,000 people in the United States in the year 2015.
Women are two-thirds more likely than men to develop irritable bowel syndrome. This may be attributable to hormonal changes related to their menstrual cycles.