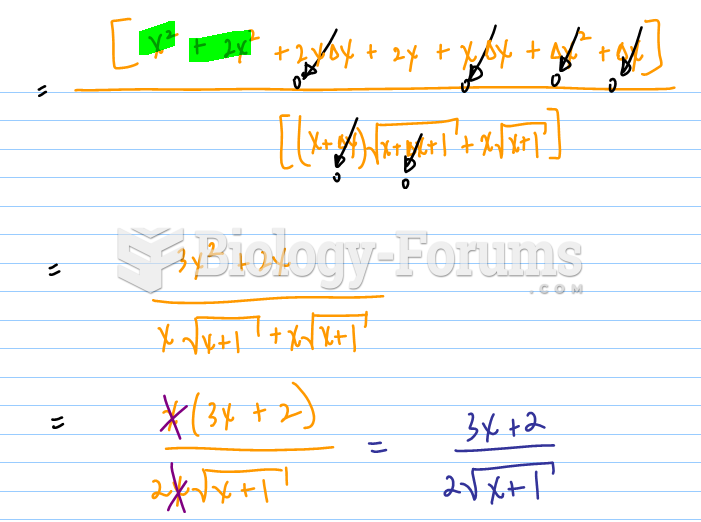
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Between 1999 and 2012, American adults with high total cholesterol decreased from 18.3% to 12.9%
Did you know?
Aspirin may benefit 11 different cancers, including those of the colon, pancreas, lungs, prostate, breasts, and leukemia.
Did you know?
Blood is approximately twice as thick as water because of the cells and other components found in it.
Did you know?
For high blood pressure (hypertension), a new class of drug, called a vasopeptidase blocker (inhibitor), has been developed. It decreases blood pressure by simultaneously dilating the peripheral arteries and increasing the body's loss of salt.
Did you know?
In 1844, Charles Goodyear obtained the first patent for a rubber condom.