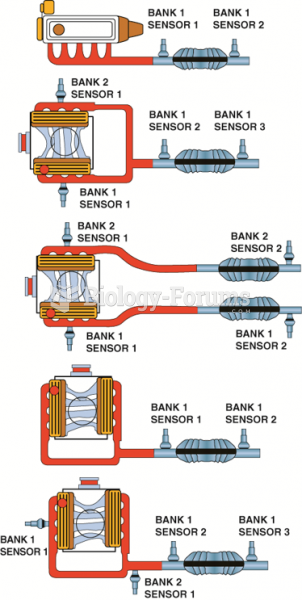
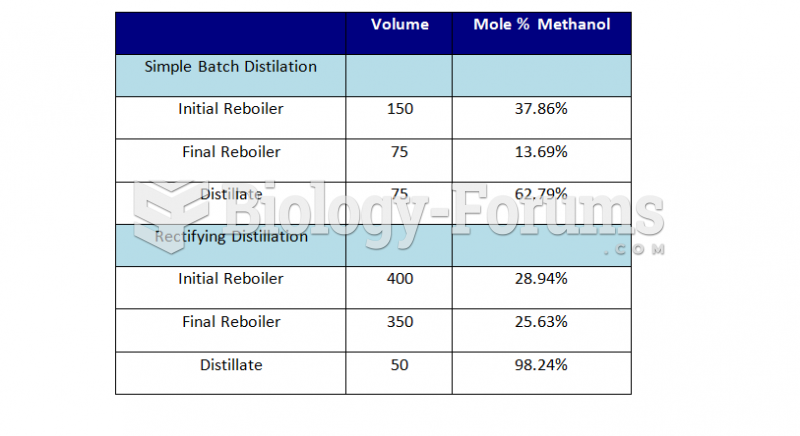
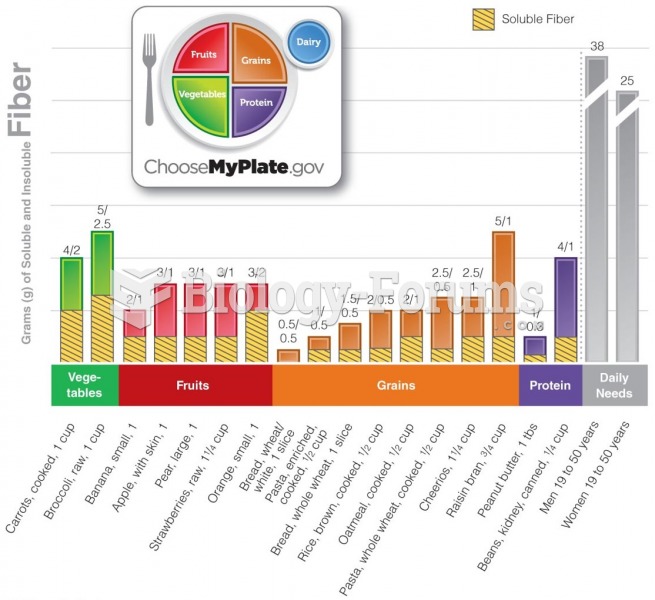
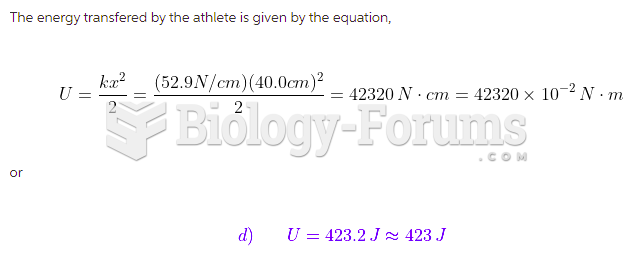
|
|
|
Everyone has one nostril that is larger than the other.
The first oral chemotherapy drug for colon cancer was approved by FDA in 2001.
The first monoclonal antibodies were made exclusively from mouse cells. Some are now fully human, which means they are likely to be safer and may be more effective than older monoclonal antibodies.
There are major differences in the metabolism of morphine and the illegal drug heroin. Morphine mostly produces its CNS effects through m-receptors, and at k- and d-receptors. Heroin has a slight affinity for opiate receptors. Most of its actions are due to metabolism to active metabolites (6-acetylmorphine, morphine, and morphine-6-glucuronide).
Russia has the highest death rate from cardiovascular disease followed by the Ukraine, Romania, Hungary, and Poland.