 |
| Previous Image | Next Image |
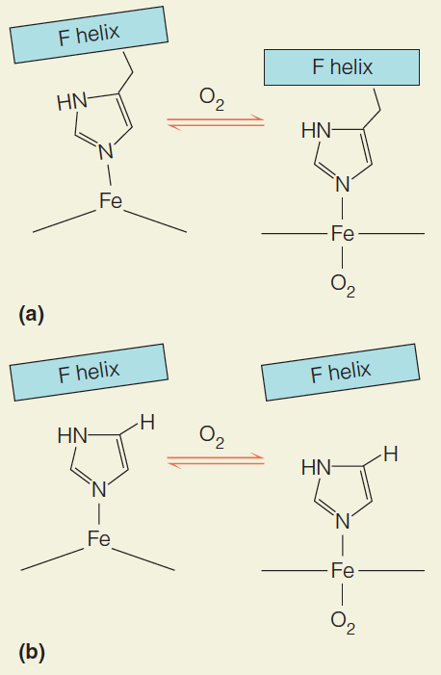
| Description: The effect of replacing the proximal histidine in hemoglobin with a glycine residue and adding a noncovalently bonded imidazole. The effect of O2 binding according to the Perutz model: the F helix is drawn toward the heme. Now lacking a connection to the heme, the F helix is not disturbed by O2 binding and there is significantly reduced cooperativity has a dome shape. Binding of the O2 ligand pulls the iron into the heme plane, flattening the heme and causing strain. A shift in the orientation of His F8 relieves the strain, partly because Val FG5 is pushed to the right. In this way, the tertiary change in heme is communicated to the FG corner. Picture Stats: Views: 303 Filesize: 74.92kB Height: 675 Width: 441 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=34127 |
