 |
| Previous Image | Next Image |
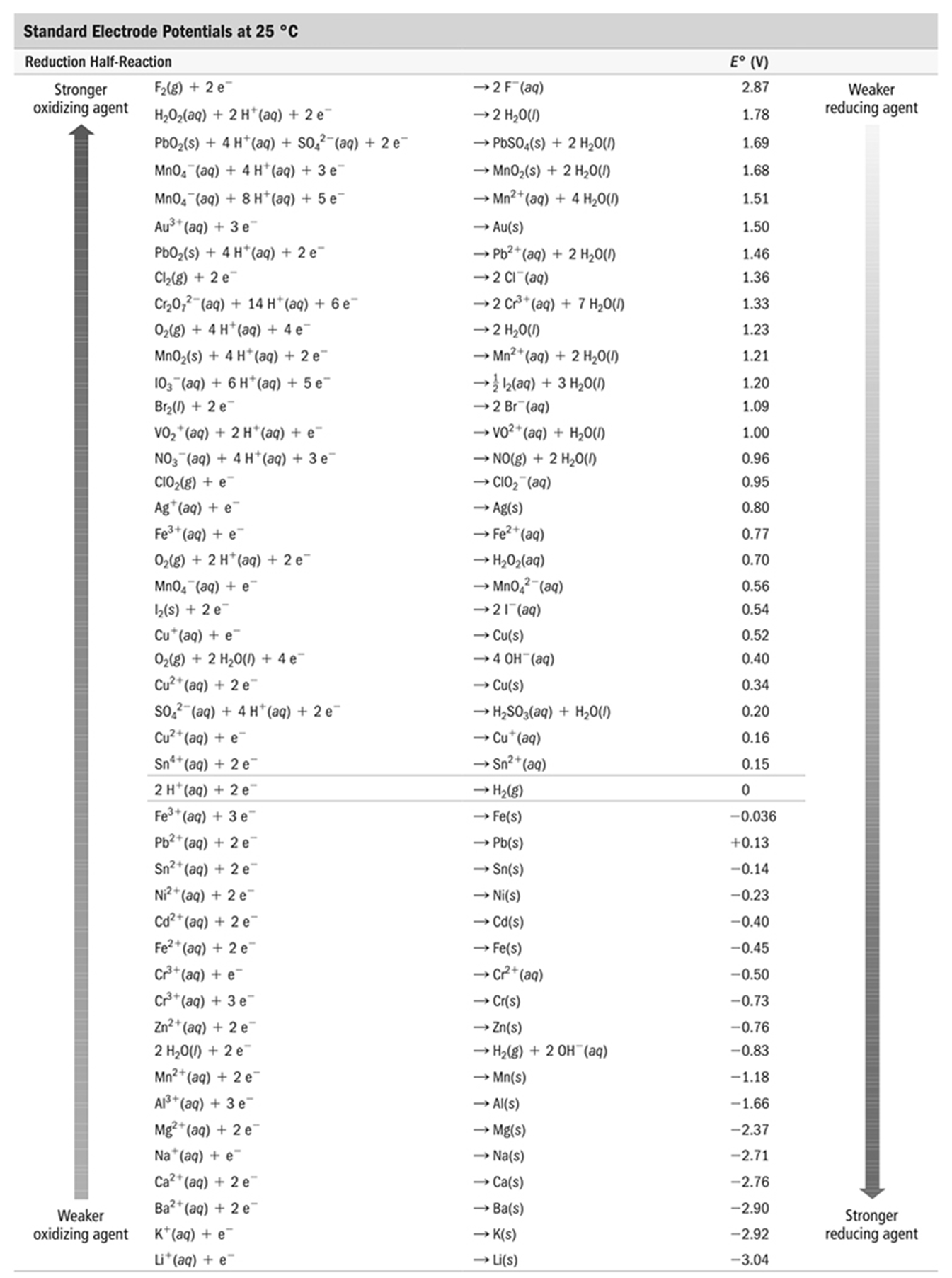
| Description: Calculating Standard Potentials for Electrochemical Cells from Standard Electrode Potentials of the Half-Reactions sum-u Moth Mullah £25 '0 Reduction Hall-Reaction Stronger W) + 2 9" oxidizing agent Weaker oxidizing agent "202030) + 2 WW) + 2 e’ mas) + 4 Wm) + $0.71”) + 2 e' MnO.‘(aq) + 4H'(aq) + 3:“ Mnoflaa) + 8H'(8¢1) + 5e' Au3‘(aq) + 32’ ”02(5) + 4n'(aq) + 29' 0,“) + 2e' 020,71”) + 14 H’(aq) 4» 6e' 02(3) + 4H‘iaa) + 4:" Mn0,(s) + 4H’(aq) + 2e‘ I0,‘(aq) + 6N’(aq) + 5c“ 372(1) + 2e’ V02'(aq) + 2H’(aa) + e“ N031“) + 4 Wm) + 3e' 002(5) + 9" WM) + 9' ré'iao) + e’ 0,(g) + 2N'(aq) + 2c" worm) + e“ I,(s) + 2e' CU'W) + 2' 01(3) + 2 H200) + 49' Cu”(aq) + 29" 30.11.21) + 4H‘(W) + 2e' Cu"(aa) + e' Sn"(aq) + 2e” 2 H'(aq) + 2 e“ Fe"(aq) + 3e‘ Pb"(aa) + 2 e' Sumac) + 2‘" Ni"(ao) + 2e' Cd"(aq) + 2e‘ Fe"(aq) + 2e’ 015160) + e" 013%“) + 3e“ Zn"(aq) + 2 e“ 2 H,0(I) + 2 e“ Mn"(aq) + 2 e' Al"(aq) + 32' Maz‘iaa) + 2e' Na‘iaa) + e' ca"(aq) + Ze‘ Ba”(ao) + 2e‘ K'(aq) + 2‘ Wm) + e' —- 2 Hail) —’ 2 H,0(I) —’ "2505(  + 2 "20(1) -. Mn0,(s) + 2 H200) —> Mn"(aa) + 4 H201!) —- Au(s) ~ Pb’wao) + 2 H200) ~ 2 c1 ' (ac) —- 2 cmaq) + 7 "200) ~ 2 H201!) —> Mn"(aa) + 2 H201!) -' i use) + 3 +4.00) -’ 2 Br'iaa) — W’iaa) + H1010 ~ mm + 2 mom —’ Clo: " (80) —’ Axis) —’ Fe‘ ‘ (an) —’ "202090) —’ Mn01’“ (ea) —’ 2 Fiat!) —~ Cu(s) —~ 6 0H '(80) —r Cu(s) -’ "250390) + H200) -' Cu 130) - Sn”(ao) —' "2(3) —. Fe(s) —~ PMs) -> Sn($) —- Ni(s) ~ 011(5) ~ Fe(s) —~ 0-2 ‘(aai —> Cris) —’ Zn(s) —- H202) + 2 0H“(aa) —~ Mn(s) ~ Alis) —. Mgis) —u Na(s) —r ca(s) —> Ba(s) —’ K(s) _. U(s) 6" (V) 2.81 L78 1.69 1.68 1.51 1.50 1.46 1.36 1.33 1.23 1.21 120 1.09 1.00 0.96 0.95 0.80 0.7 7 0.70 0.56 0.54 0.52 0.40 0.34 0.20 0.16 0. i 5 -0.036 +0.13 -0.14 -0.23 ~0.40 —0.45 —0.50 -0.73 —0.76 —0.83 -1.18 -1.66 -2.37 -2.71 ~2.76 -2.90 -2.92 -3.04 Weaket redudng agent Strange! reducing agent + 2 "20(1) -. Mn0,(s) + 2 H200) —> Mn"(aa) + 4 H201!) —- Au(s) ~ Pb’wao) + 2 H200) ~ 2 c1 ' (ac) —- 2 cmaq) + 7 "200) ~ 2 H201!) —> Mn"(aa) + 2 H201!) -' i use) + 3 +4.00) -’ 2 Br'iaa) — W’iaa) + H1010 ~ mm + 2 mom —’ Clo: " (80) —’ Axis) —’ Fe‘ ‘ (an) —’ "202090) —’ Mn01’“ (ea) —’ 2 Fiat!) —~ Cu(s) —~ 6 0H '(80) —r Cu(s) -’ "250390) + H200) -' Cu 130) - Sn”(ao) —' "2(3) —. Fe(s) —~ PMs) -> Sn($) —- Ni(s) ~ 011(5) ~ Fe(s) —~ 0-2 ‘(aai —> Cris) —’ Zn(s) —- H202) + 2 0H“(aa) —~ Mn(s) ~ Alis) —. Mgis) —u Na(s) —r ca(s) —> Ba(s) —’ K(s) _. U(s) 6" (V) 2.81 L78 1.69 1.68 1.51 1.50 1.46 1.36 1.33 1.23 1.21 120 1.09 1.00 0.96 0.95 0.80 0.7 7 0.70 0.56 0.54 0.52 0.40 0.34 0.20 0.16 0. i 5 -0.036 +0.13 -0.14 -0.23 ~0.40 —0.45 —0.50 -0.73 —0.76 —0.83 -1.18 -1.66 -2.37 -2.71 ~2.76 -2.90 -2.92 -3.04 Weaket redudng agent Strange! reducing agent
Picture Stats: Views: 926 Filesize: 575.1kB Height: 1805 Width: 1345 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=45835 |
