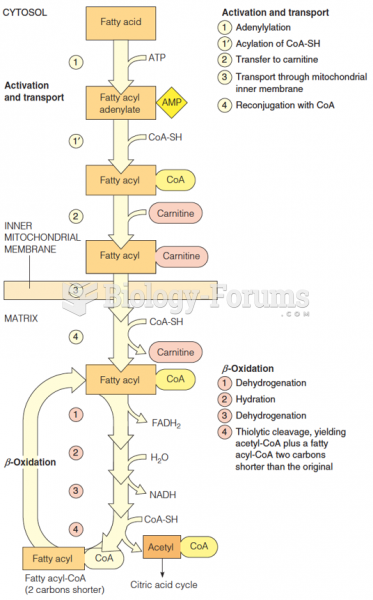
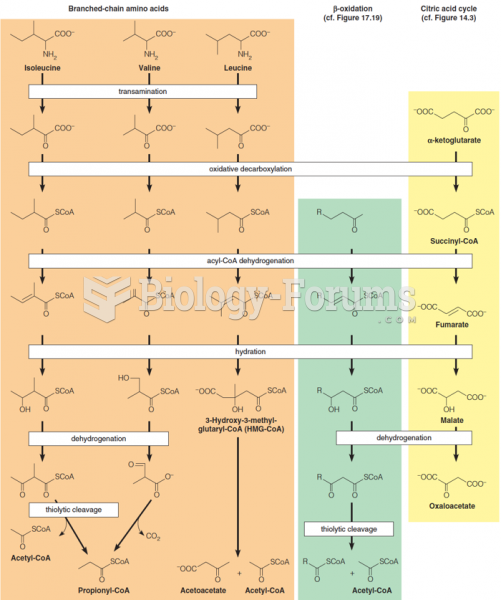
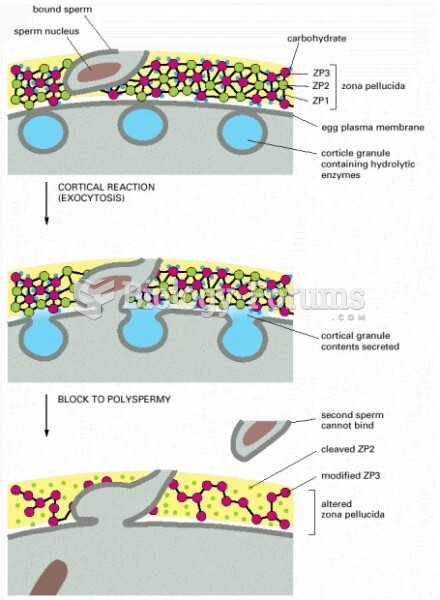
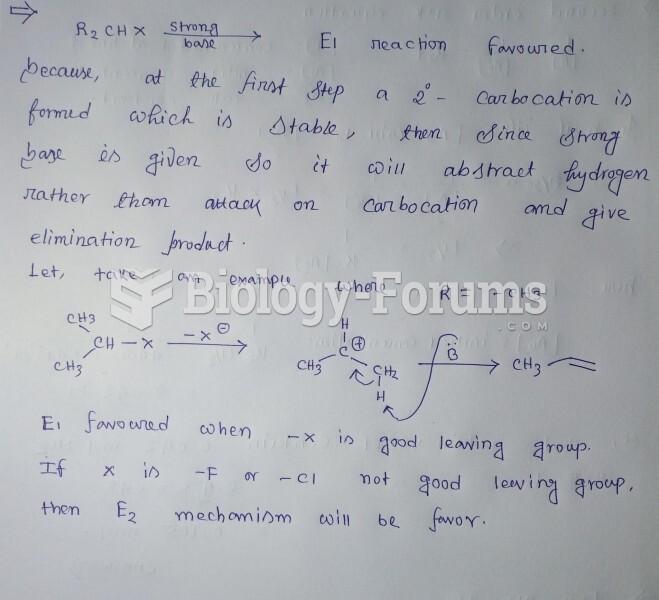
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.
Did you know?
A seasonal flu vaccine is the best way to reduce the chances you will get seasonal influenza and spread it to others.
Did you know?
Russia has the highest death rate from cardiovascular disease followed by the Ukraine, Romania, Hungary, and Poland.
Did you know?
As many as 20% of Americans have been infected by the fungus known as Histoplasmosis. While most people are asymptomatic or only have slight symptoms, infection can progress to a rapid and potentially fatal superinfection.
Did you know?
Excessive alcohol use costs the country approximately $235 billion every year.