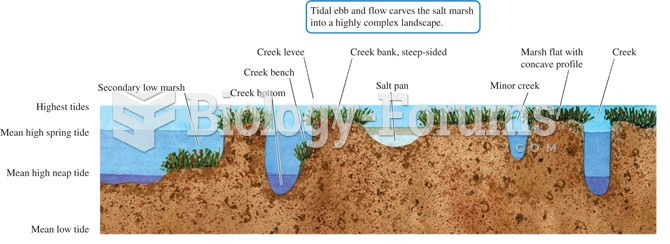
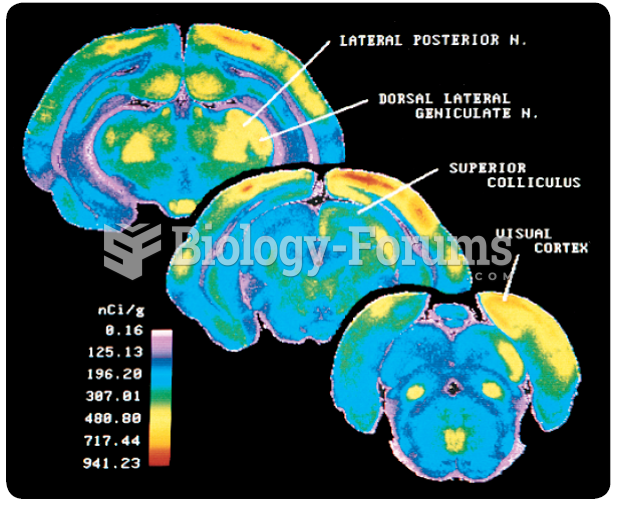
|
|
|
IgA antibodies protect body surfaces exposed to outside foreign substances. IgG antibodies are found in all body fluids. IgM antibodies are the first type of antibody made in response to an infection. IgE antibody levels are often high in people with allergies. IgD antibodies are found in tissues lining the abdomen and chest.
Looking at the sun may not only cause headache and distort your vision temporarily, but it can also cause permanent eye damage. Any exposure to sunlight adds to the cumulative effects of ultraviolet (UV) radiation on your eyes. UV exposure has been linked to eye disorders such as macular degeneration, solar retinitis, and corneal dystrophies.
Increased intake of vitamin D has been shown to reduce fractures up to 25% in older people.
Fatal fungal infections may be able to resist newer antifungal drugs. Globally, fungal infections are often fatal due to the lack of access to multiple antifungals, which may be required to be utilized in combination. Single antifungals may not be enough to stop a fungal infection from causing the death of a patient.
Pregnant women usually experience a heightened sense of smell beginning late in the first trimester. Some experts call this the body's way of protecting a pregnant woman from foods that are unsafe for the fetus.
 That politics was always a rough business is shown in this cartoon, which shows Lincoln, assisted by
That politics was always a rough business is shown in this cartoon, which shows Lincoln, assisted by
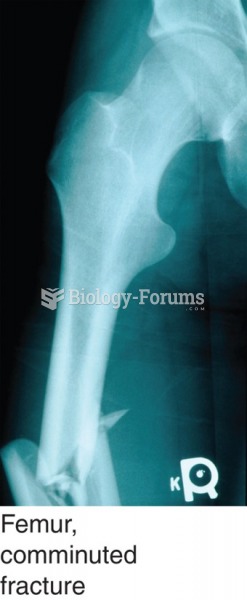 Fractures are diagnosed based on an X-ray of the broken bone. Shown here: a comminuted fracture of ...
Fractures are diagnosed based on an X-ray of the broken bone. Shown here: a comminuted fracture of ...