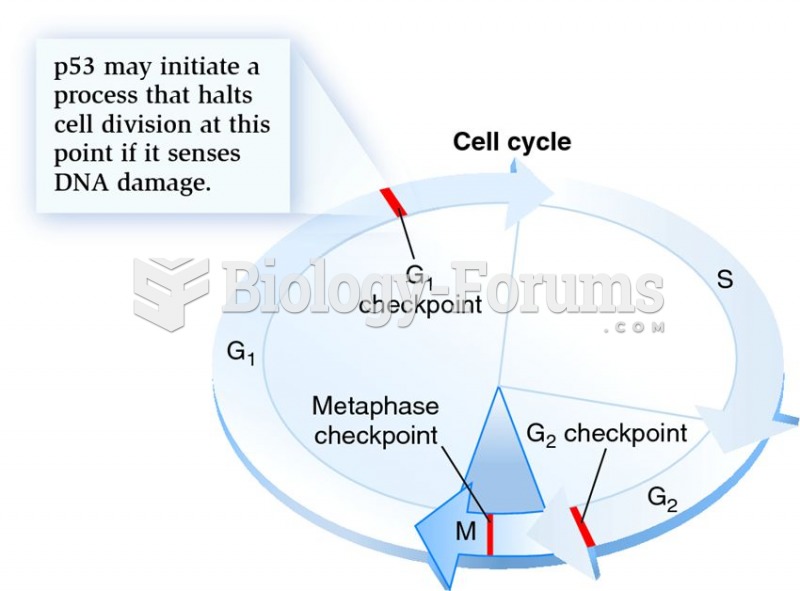
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Autoimmune diseases occur when the immune system destroys its own healthy tissues. When this occurs, white blood cells cannot distinguish between pathogens and normal cells.
Did you know?
The average older adult in the United States takes five prescription drugs per day. Half of these drugs contain a sedative. Alcohol should therefore be avoided by most senior citizens because of the dangerous interactions between alcohol and sedatives.
Did you know?
Human kidneys will clean about 1 million gallons of blood in an average lifetime.
Did you know?
Atropine was named after the Greek goddess Atropos, the oldest and ugliest of the three sisters known as the Fates, who controlled the destiny of men.
Did you know?
Medication errors are three times higher among children and infants than with adults.