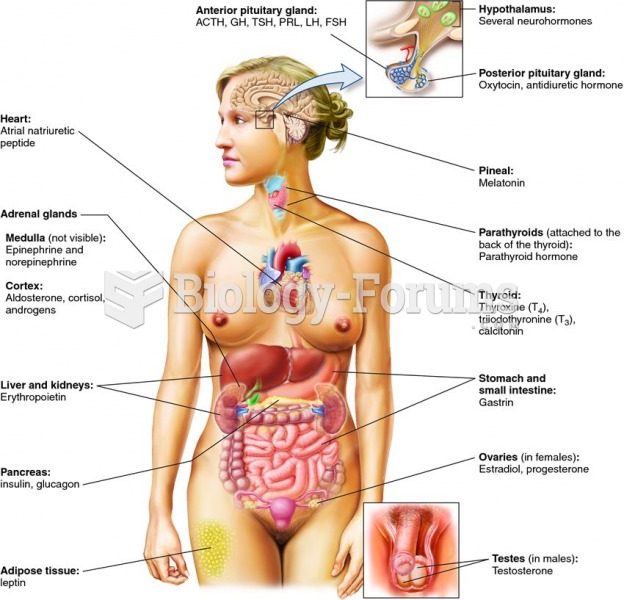
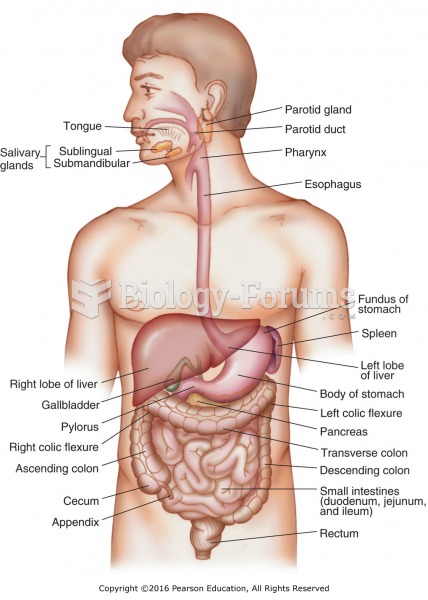
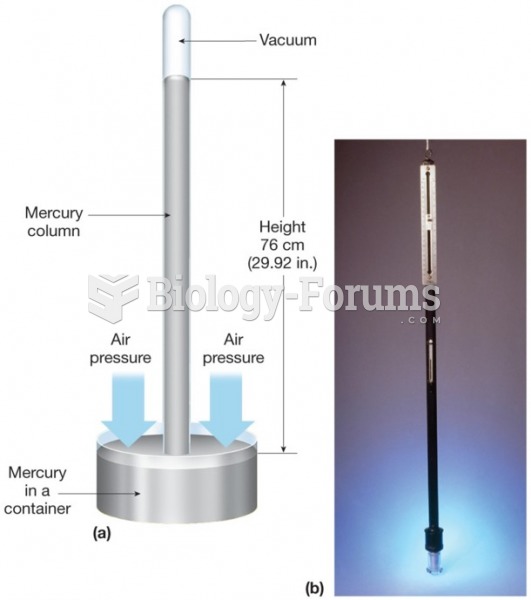
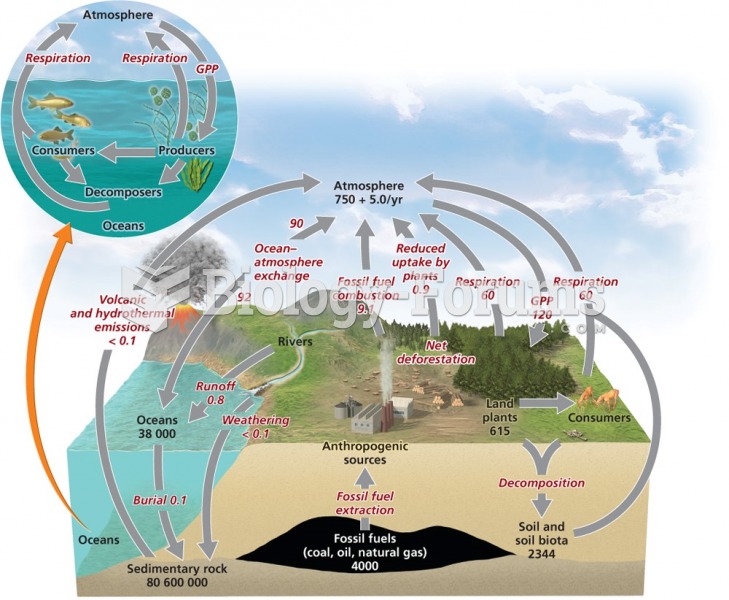
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The average human gut is home to perhaps 500 to 1,000 different species of bacteria.
Did you know?
The first oncogene was discovered in 1970 and was termed SRC (pronounced "SARK").
Did you know?
Atropine was named after the Greek goddess Atropos, the oldest and ugliest of the three sisters known as the Fates, who controlled the destiny of men.
Did you know?
Patients who have been on total parenteral nutrition for more than a few days may need to have foods gradually reintroduced to give the digestive tract time to start working again.
Did you know?
The immune system needs 9.5 hours of sleep in total darkness to recharge completely.