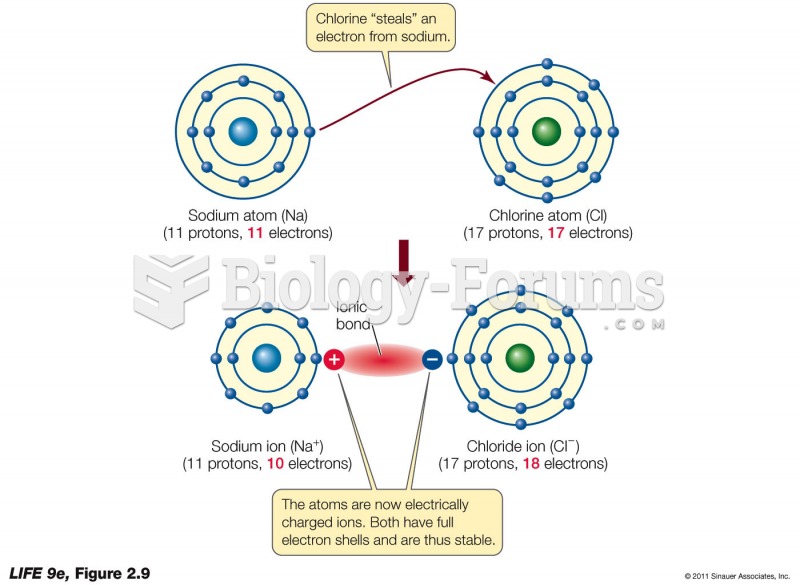
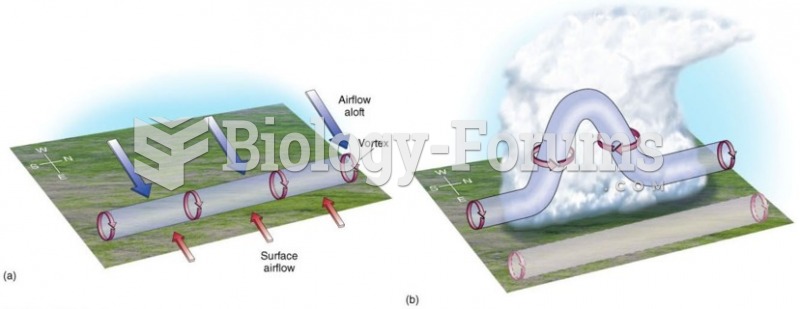
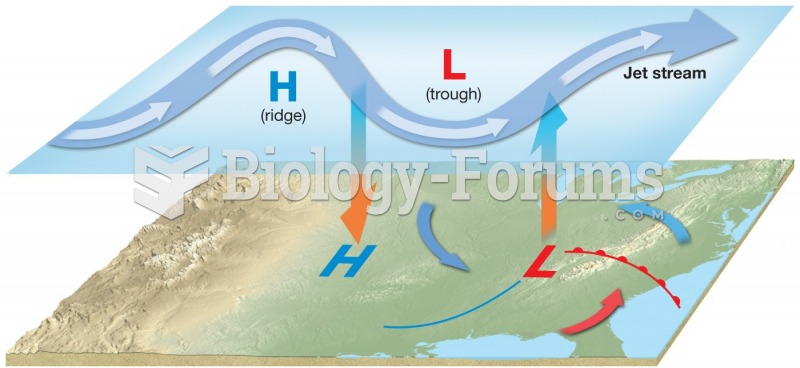
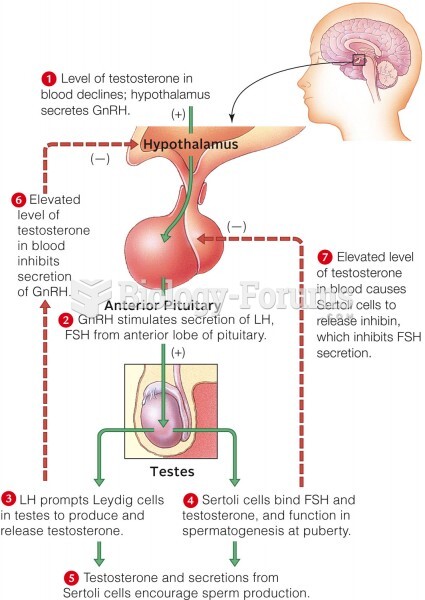
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Opium has influenced much of the world's most popular literature. The following authors were all opium users, of varying degrees: Lewis Carroll, Charles, Dickens, Arthur Conan Doyle, and Oscar Wilde.
Did you know?
More than 30% of American adults, and about 12% of children utilize health care approaches that were developed outside of conventional medicine.
Did you know?
Nitroglycerin is used to alleviate various heart-related conditions, and it is also the chief component of dynamite (but mixed in a solid clay base to stabilize it).
Did you know?
Bacteria have flourished on the earth for over three billion years. They were the first life forms on the planet.
Did you know?
Medication errors are more common among seriously ill patients than with those with minor conditions.