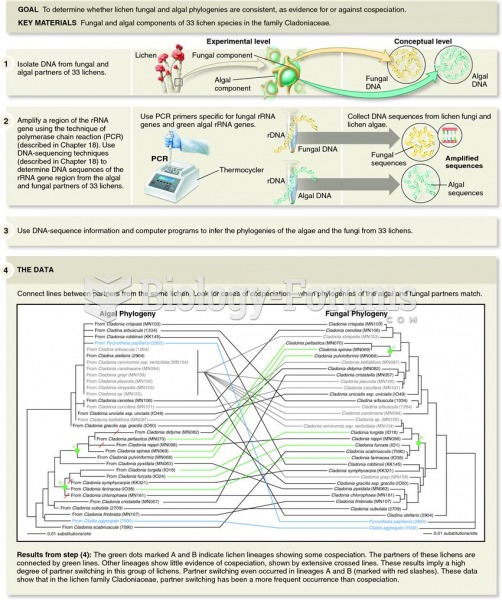
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Alcohol acts as a diuretic. Eight ounces of water is needed to metabolize just 1 ounce of alcohol.
Did you know?
Drugs are in development that may cure asthma and hay fever once and for all. They target leukotrienes, which are known to cause tightening of the air passages in the lungs and increase mucus productions in nasal passages.
Did you know?
Multiple experimental evidences have confirmed that at the molecular level, cancer is caused by lesions in cellular DNA.
Did you know?
Asthma cases in Americans are about 75% higher today than they were in 1980.
Did you know?
There are over 65,000 known species of protozoa. About 10,000 species are parasitic.