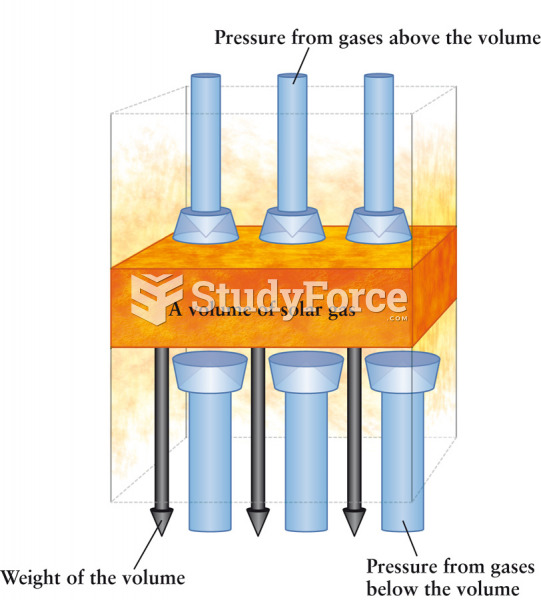
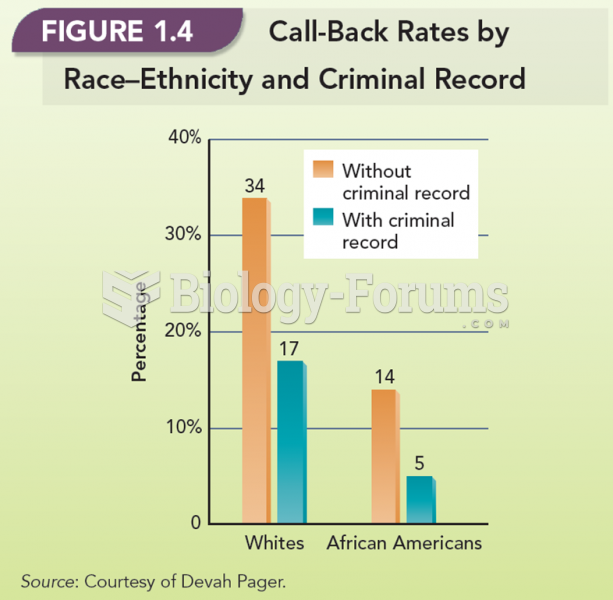
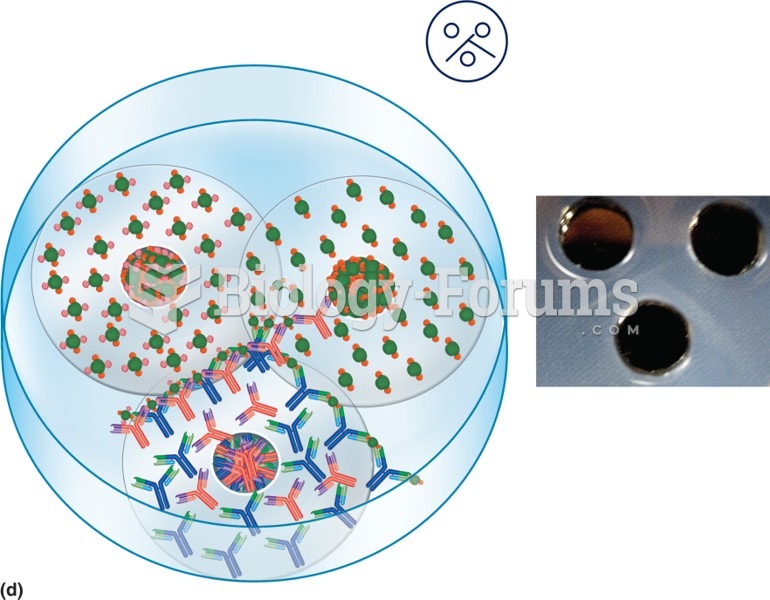
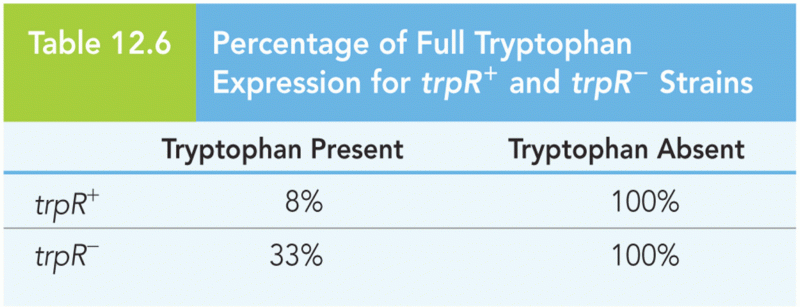
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Oliver Wendell Holmes is credited with introducing the words "anesthesia" and "anesthetic" into the English language in 1846.
Did you know?
The immune system needs 9.5 hours of sleep in total darkness to recharge completely.
Did you know?
Computer programs are available that crosscheck a new drug's possible trade name with all other trade names currently available. These programs detect dangerous similarities between names and alert the manufacturer of the drug.
Did you know?
Cyanide works by making the human body unable to use oxygen.
Did you know?
To combat osteoporosis, changes in lifestyle and diet are recommended. At-risk patients should include 1,200 to 1,500 mg of calcium daily either via dietary means or with supplements.