|
|
|
Fatal fungal infections may be able to resist newer antifungal drugs. Globally, fungal infections are often fatal due to the lack of access to multiple antifungals, which may be required to be utilized in combination. Single antifungals may not be enough to stop a fungal infection from causing the death of a patient.
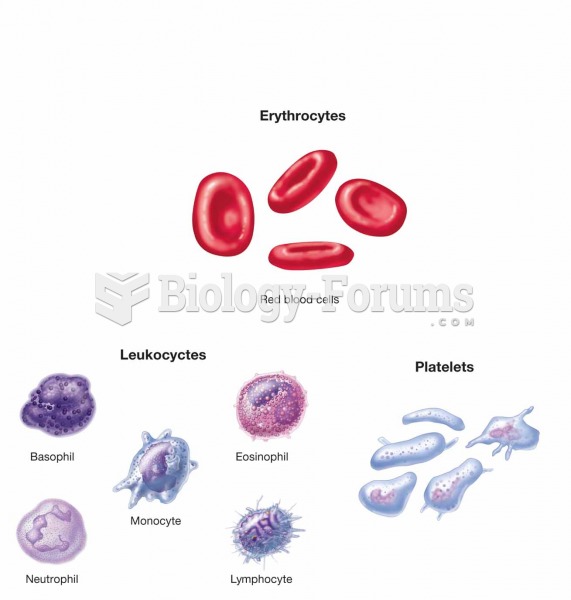
There are 20 feet of blood vessels in each square inch of human skin.
Thyroid conditions cause a higher risk of fibromyalgia and chronic fatigue syndrome.
According to the National Institute of Environmental Health Sciences, lung disease is the third leading killer in the United States, responsible for one in seven deaths. It is the leading cause of death among infants under the age of one year.
Human kidneys will clean about 1 million gallons of blood in an average lifetime.