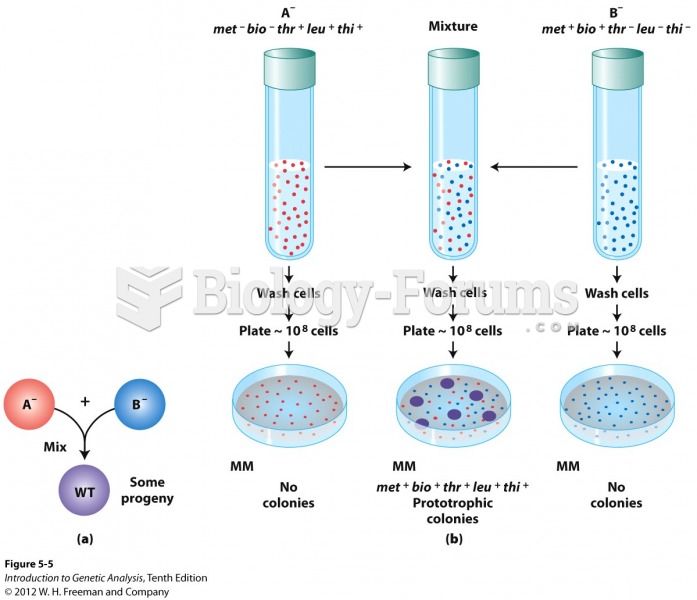
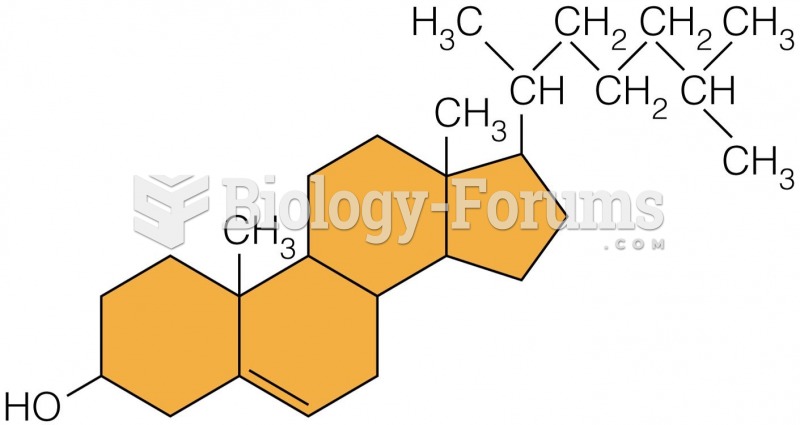
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
There are more nerve cells in one human brain than there are stars in the Milky Way.
Did you know?
During pregnancy, a woman is more likely to experience bleeding gums and nosebleeds caused by hormonal changes that increase blood flow to the mouth and nose.
Did you know?
It is believed that humans initially contracted crabs from gorillas about 3 million years ago from either sleeping in gorilla nests or eating the apes.
Did you know?
Serum cholesterol testing in adults is recommended every 1 to 5 years. People with diabetes and a family history of high cholesterol should be tested even more frequently.
Did you know?
More than 4.4billion prescriptions were dispensed within the United States in 2016.