This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The oldest recorded age was 122. Madame Jeanne Calment was born in France in 1875 and died in 1997. She was a vegetarian and loved olive oil, port wine, and chocolate.
Did you know?
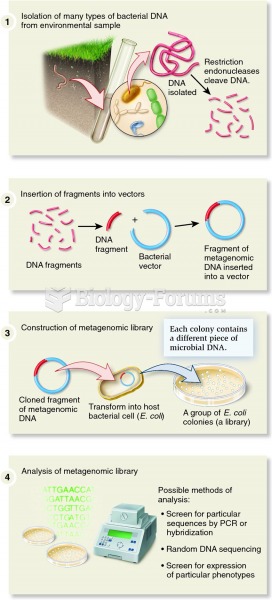
Bacteria have been found alive in a lake buried one half mile under ice in Antarctica.
Did you know?
Hyperthyroidism leads to an increased rate of metabolism and affects about 1% of women but only 0.1% of men. For most people, this increased metabolic rate causes the thyroid gland to become enlarged (known as a goiter).
Did you know?
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
Did you know?
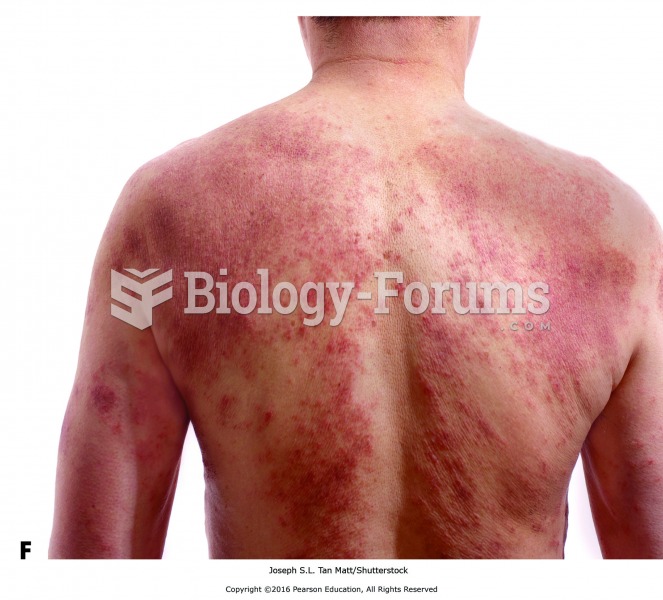
Fungal nail infections account for up to 30% of all skin infections. They affect 5% of the general population—mostly people over the age of 70.