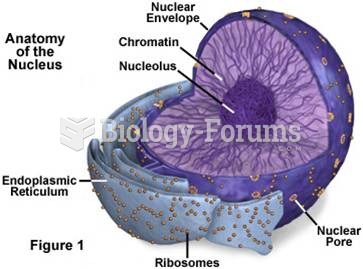
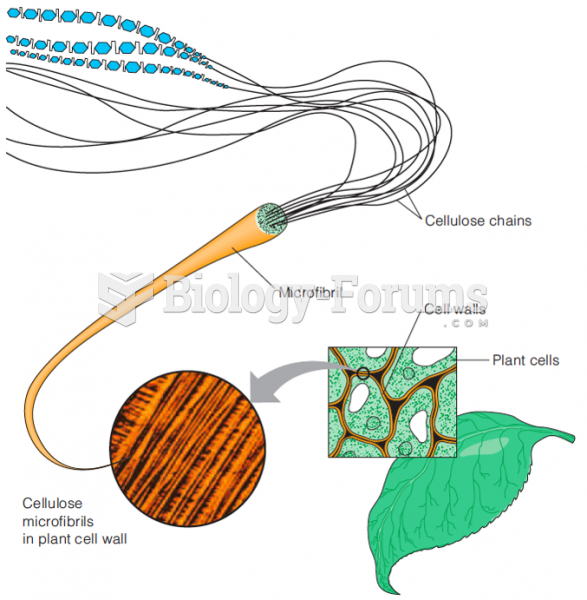
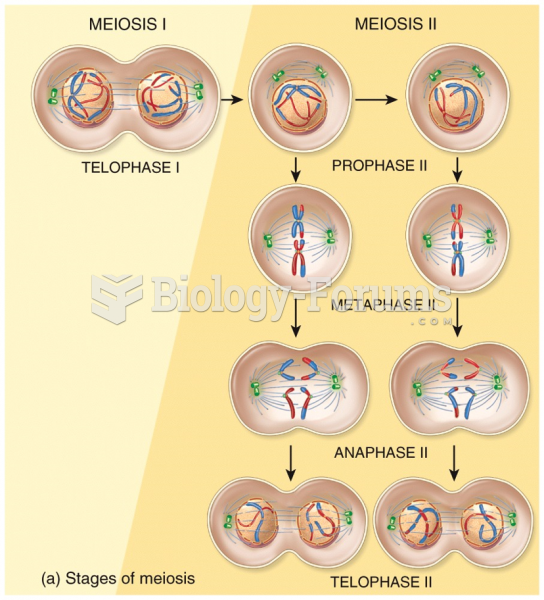
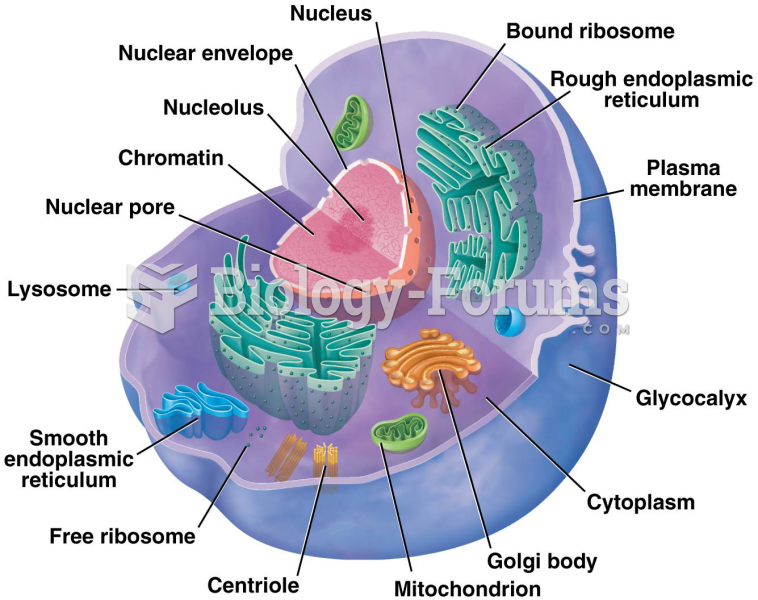
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Warfarin was developed as a consequence of the study of a strange bleeding disorder that suddenly occurred in cattle on the northern prairies of the United States in the early 1900s.
Did you know?
Cancer has been around as long as humankind, but only in the second half of the twentieth century did the number of cancer cases explode.
Did you know?
On average, someone in the United States has a stroke about every 40 seconds. This is about 795,000 people per year.
Did you know?
Normal urine is sterile. It contains fluids, salts, and waste products. It is free of bacteria, viruses, and fungi.
Did you know?
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.