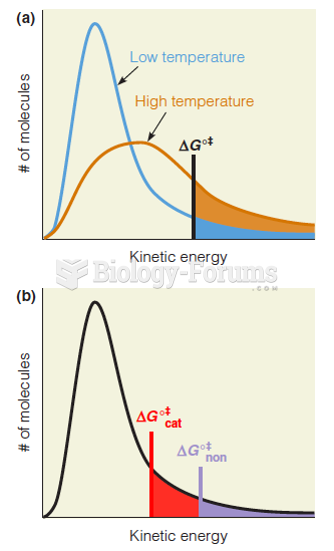
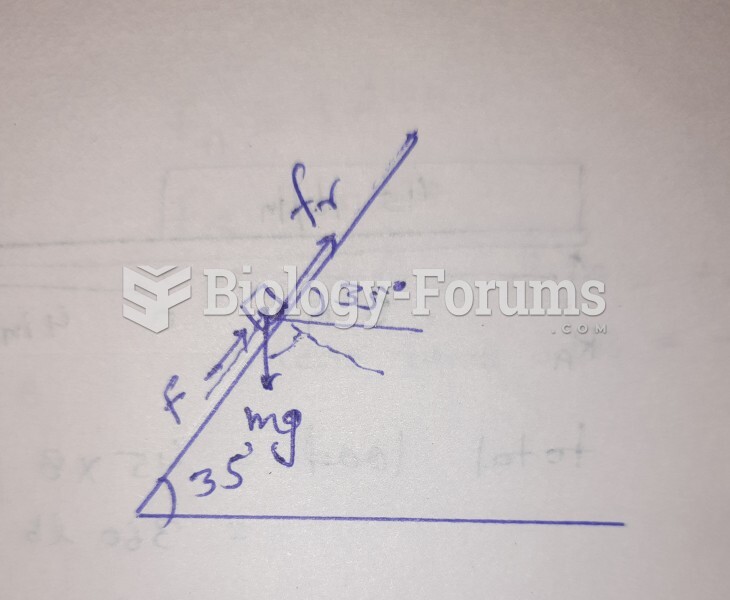
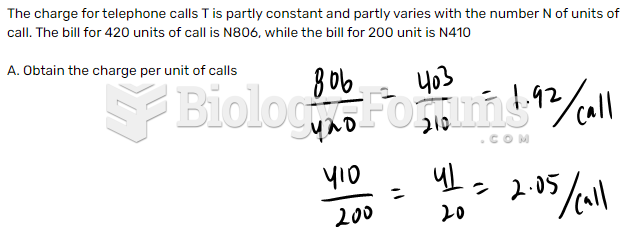This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
In 1844, Charles Goodyear obtained the first patent for a rubber condom.
Did you know?
The top five reasons that children stay home from school are as follows: colds, stomach flu (gastroenteritis), ear infection (otitis media), pink eye (conjunctivitis), and sore throat.
Did you know?
Street names for barbiturates include reds, red devils, yellow jackets, blue heavens, Christmas trees, and rainbows. They are commonly referred to as downers.
Did you know?
Green tea is able to stop the scent of garlic or onion from causing bad breath.
Did you know?
Elderly adults are living longer, and causes of death are shifting. At the same time, autopsy rates are at or near their lowest in history.