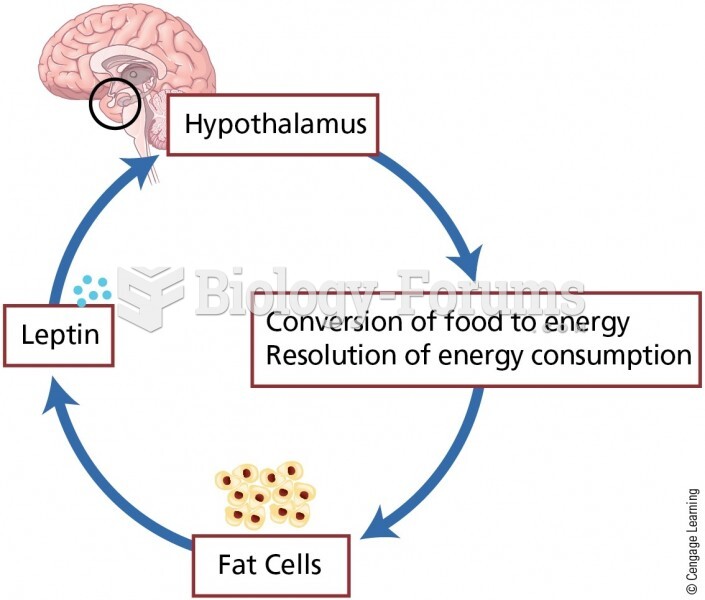
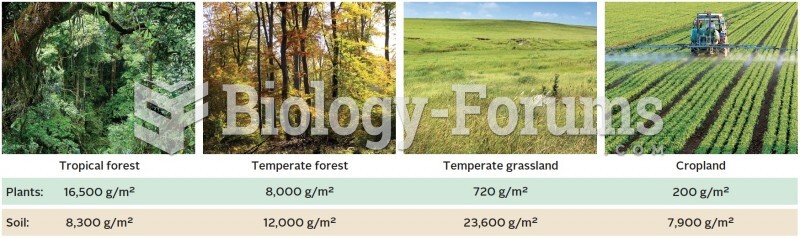
|
|
|
Approximately 25% of all reported medication errors result from some kind of name confusion.
All adverse reactions are commonly charted in red ink in the patient's record and usually are noted on the front of the chart. Failure to follow correct documentation procedures may result in malpractice lawsuits.
Blood in the urine can be a sign of a kidney stone, glomerulonephritis, or other kidney problems.
The top 10 most important tips that will help you grow old gracefully include (1) quit smoking, (2) keep your weight down, (3) take supplements, (4) skip a meal each day or fast 1 day per week, (5) get a pet, (6) get medical help for chronic pain, (7) walk regularly, (8) reduce arguments, (9) put live plants in your living space, and (10) do some weight training.
More than 30% of American adults, and about 12% of children utilize health care approaches that were developed outside of conventional medicine.