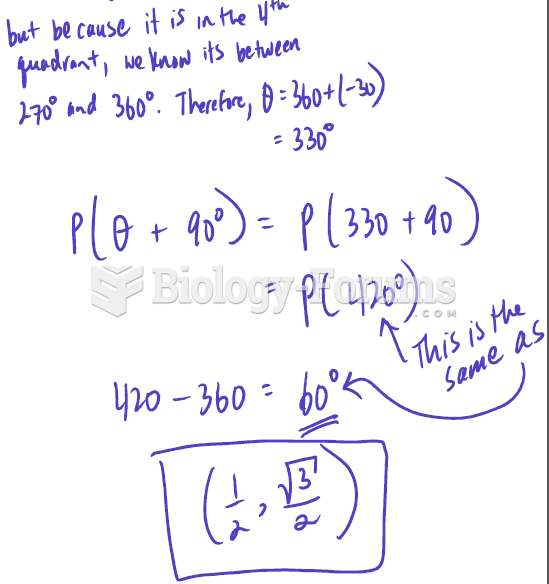
|
|
|
Patients should never assume they are being given the appropriate drugs. They should make sure they know which drugs are being prescribed, and always double-check that the drugs received match the prescription.
Blood is approximately twice as thick as water because of the cells and other components found in it.
Certain rare plants containing cyanide include apricot pits and a type of potato called cassava. Fortunately, only chronic or massive ingestion of any of these plants can lead to serious poisoning.
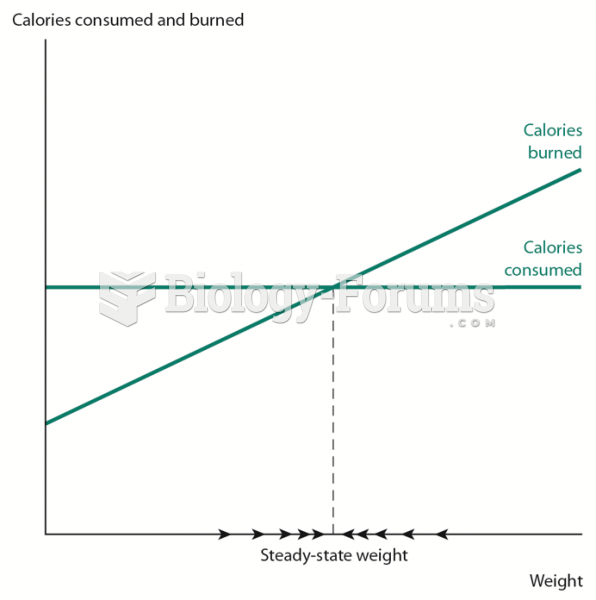
More than one-third of adult Americans are obese. Diseases that kill the largest number of people annually, such as heart disease, cancer, diabetes, stroke, and hypertension, can be attributed to diet.
Earwax has antimicrobial properties that reduce the viability of bacteria and fungus in the human ear.




![Given the values of sin θ, determine the values of θ over the interval [0, 2pi]](https://biology-forums.com/gallery/42/medium_6_04_01_21_12_54_15.png)