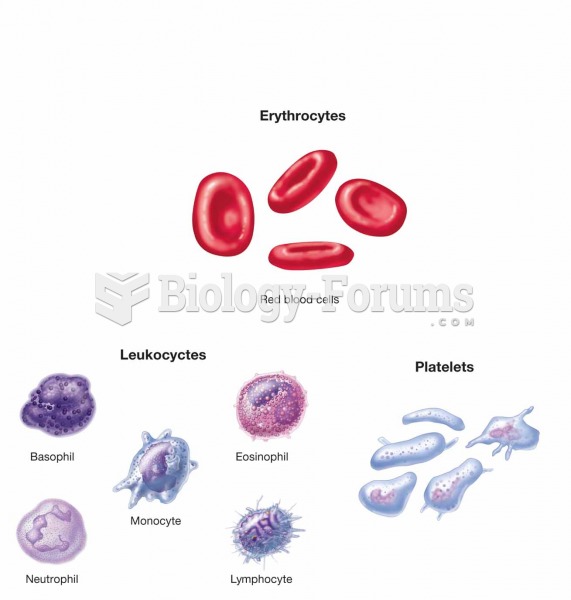
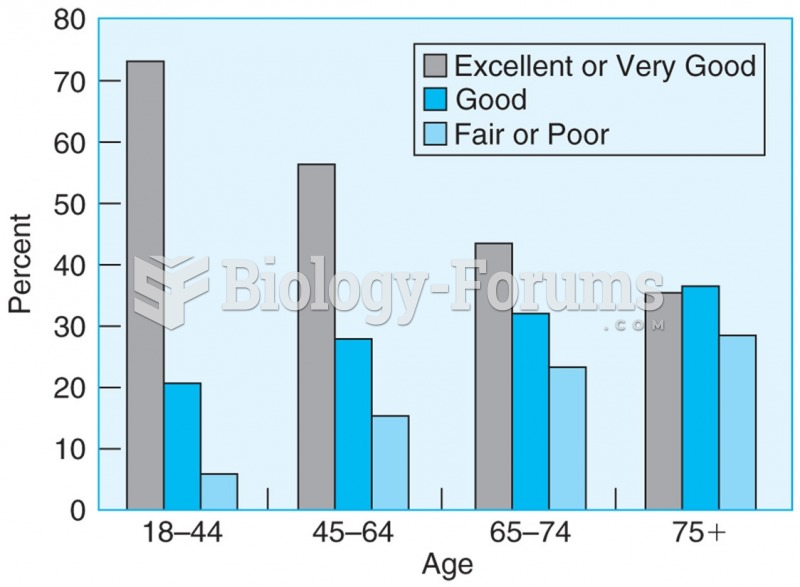
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Everyone has one nostril that is larger than the other.
Did you know?
Cyanide works by making the human body unable to use oxygen.
Did you know?
Most strokes are caused when blood clots move to a blood vessel in the brain and block blood flow to that area. Thrombolytic therapy can be used to dissolve the clot quickly. If given within 3 hours of the first stroke symptoms, this therapy can help limit stroke damage and disability.
Did you know?
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.
Did you know?
A seasonal flu vaccine is the best way to reduce the chances you will get seasonal influenza and spread it to others.