|
|
|
Russia has the highest death rate from cardiovascular disease followed by the Ukraine, Romania, Hungary, and Poland.
Patients who have undergone chemotherapy for the treatment of cancer often complain of a lack of mental focus; memory loss; and a general diminution in abilities such as multitasking, attention span, and general mental agility.
Of the estimated 2 million heroin users in the United States, 600,000–800,000 are considered hardcore addicts. Heroin addiction is considered to be one of the hardest addictions to recover from.
Although the Roman numeral for the number 4 has always been taught to have been "IV," according to historians, the ancient Romans probably used "IIII" most of the time. This is partially backed up by the fact that early grandfather clocks displayed IIII for the number 4 instead of IV. Early clockmakers apparently thought that the IIII balanced out the VIII (used for the number 8) on the clock face and that it just looked better.
Cyanide works by making the human body unable to use oxygen.
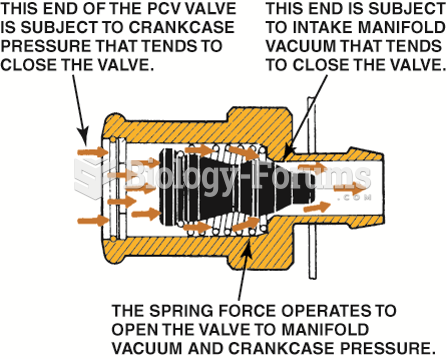 Spring force, crankcase pressure, and intake manifold vacuum work together to regulate the flow rate ...
Spring force, crankcase pressure, and intake manifold vacuum work together to regulate the flow rate ...
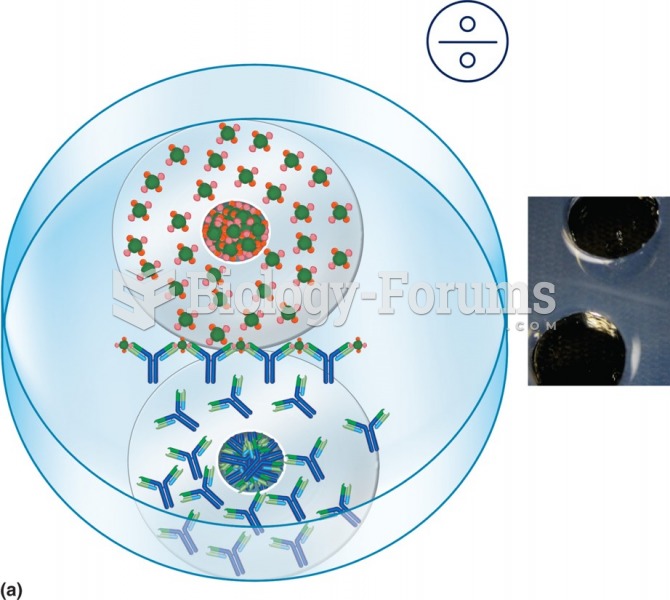 An Ouchterlony double diffusion gel precipitation assay. The antigen and antibody solutions are most ...
An Ouchterlony double diffusion gel precipitation assay. The antigen and antibody solutions are most ...