This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
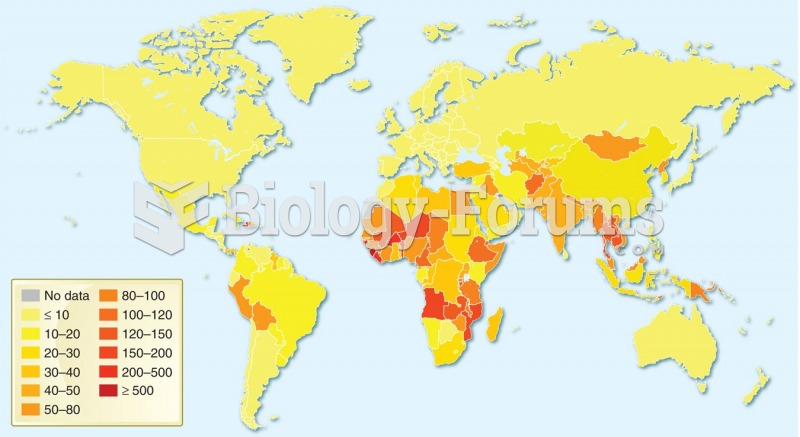
Illness; diuretics; laxative abuse; hot weather; exercise; sweating; caffeine; alcoholic beverages; starvation diets; inadequate carbohydrate consumption; and diets high in protein, salt, or fiber can cause people to become dehydrated.
Did you know?
Women are 50% to 75% more likely than men to experience an adverse drug reaction.
Did you know?
Always store hazardous household chemicals in their original containers out of reach of children. These include bleach, paint, strippers and products containing turpentine, garden chemicals, oven cleaners, fondue fuels, nail polish, and nail polish remover.
Did you know?
The immune system needs 9.5 hours of sleep in total darkness to recharge completely.
Did you know?
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.